La Crosse virus (LACV) is an arthropod-borne virus (arbovirus) of the California encephalitis serogroup of Orthobunyaviruses (family Peribunyaviridae).1,2 La Crosse virus was originally isolated in 1964 from the brain tissue of a child in La Crosse, Wisconsin, who died in 1960 from a then-undiagnosed, yet fatal, neuroinvasive illness.3 There is evidence that LACV disease (initially reported as “California Virus Encephalitis”) was present in North Carolina in 1964, coincident with the discovery of LACV in Wisconsin. In the years following its discovery, LACV disease was more frequently reported within Midwestern states. In contrast, there were only eight cases of LACVND reported in North Carolina from 1964 to 1977; all cases were children (aged 4–12 years) with residences in or prior travel to Western North Carolina or, more specifically, the Great Smoky Mountains National Park.4 However, over the past two decades, LACV disease has increasingly been reported throughout the Appalachian region with the majority of human cases occurring in Ohio, North Carolina, Tennessee, and West Virginia.5,6 It is unknown whether this increased incidence in the Appalachian region is due to changes in the distribution of LACV, changes in the abundance of vectors (including two non-native LACV vector species—Aedes japonicus and Aedes albopictus—that have been introduced in recent decades), increased clinical recognition, a changing climate, population growth, or some combination of these factors. Recent evidence further suggests that LACVND is geographically persistent with statistically significant high-risk clustering occurring at the county level within these states.7
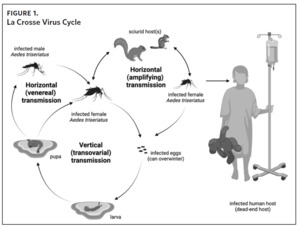
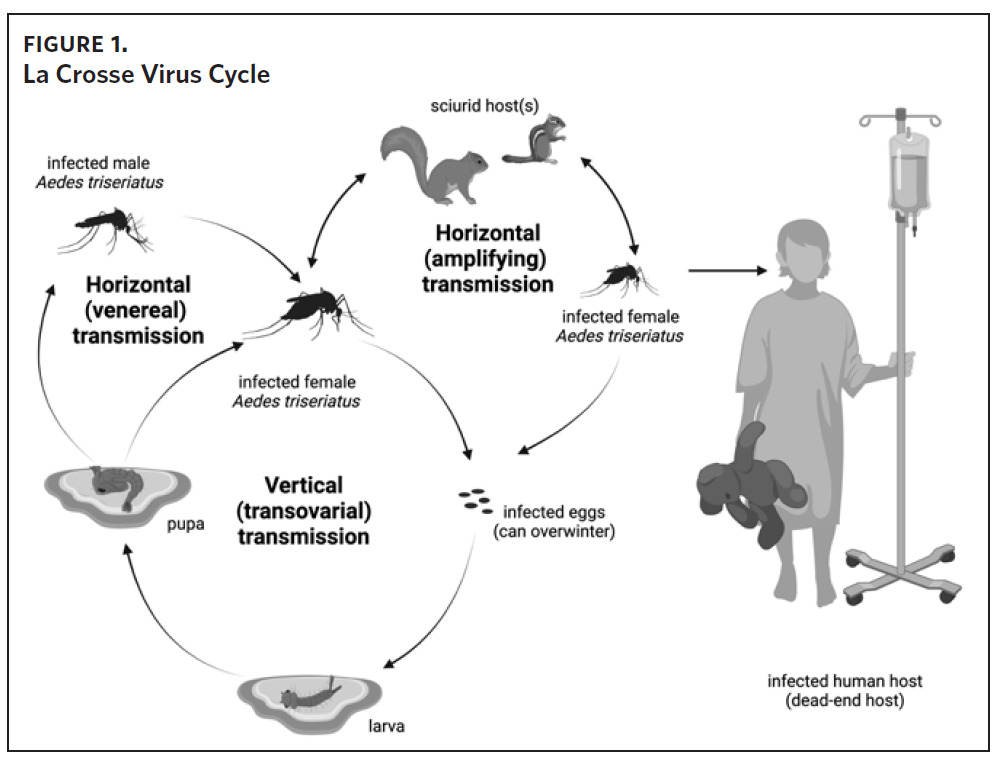
La Crosse virus is transmitted to humans through the bite of infectious female mosquitoes, primarily the eastern tree-hole mosquito (Aedes triseriatus). The natural history of LACV is complex (Figure 1). In mosquitoes, LACV can be transmitted by one of three mechanisms: 1) from female mosquito to her offspring (vertical transmission), 2) by means of amplifying rodent hosts such as chipmunks and gray squirrels (horizontal transmission), 3) and from an infected male mosquito to uninfected female mosquito during mating (venereal horizontal transmission).8,9 La Crosse virus also overwinters from one year to the next in infected mosquito eggs, creating the potential for foci with persistent infection risk.9,10 Humans are dead-end hosts (i.e., they do not develop sufficient viremias to infect other mosquitoes) that are exclusively infected by the bite of a mosquito.11
The burden of LACVND is primarily borne by children, with symptomatic cases predominantly occurring in children under age 16.6 Neuroinvasive LACV disease typically manifests as encephalitis, which may be complicated by lethargy, seizures, coma, and rarely death (case fatality rate: 1%–2%).5,6,12,13 However, severe neuroinvasive disease represents a minority of LACV exposures, as most infections are likely asymptomatic or mild,14 and do not prompt care-seeking and diagnostic testing. For example, it is estimated that for every neuroinvasive LACV disease case, there are 100 to 300 unrecognized LACV infections.12,14 A seroprevalence study of more than 1000 individuals in Western North Carolina conducted during 1989–1990 demonstrated that approximately 10% of those sampled had neutralizing antibodies to LACV, with the highest rates observed among Native American populations.15 Unfortunately, more recent estimates that might reflect the impacts of population growth or a changing climate are not currently available.
At present, there are no specific antiviral medications to treat or vaccines to prevent LACV disease. Clinical care is largely supportive, although no specific interventions (e.g., antipyretics, antiepileptics) have been shown to improve outcomes.12,16 While the case fatality rate is low (< 1%), severe neuroinvasive cases often require critical care services such as mechanical ventilation that are only available in an intensive care unit; these services are often unavailable in the rural communities where the disease is endemic.17 Long-term, post-infectious sequelae include seizures, cognitive impairment and delays, neuromotor dysfunction, and behavioral changes.12,13,17 The prevalence and burdens of long-term LACVND sequelae remain enigmatic and are likely underreported. A recent 10-year retrospective study of LACVND by Boutzoukas and colleagues (2023) reported that 35% of respondents (median time from hospital discharge to study recruitment: 4.3 years [IQR, 1.4–7.4 years]) received special education services, including Individual Education Plans or Section 504 Plans, suggesting that these children may have persistent physical or cognitive impairments limiting one or more major life activities.17 Similarly, the full social and economic impacts of LACV are unclear, but also likely underappreciated. A 2003 study conducted in Western North Carolina found that the estimated total financial impact (direct and indirect medical costs) was $794,303 (~1.3 million in 2023 US dollars) over 89.6 life years (n = 24 LACVND cases) from the onset of illness to the date of interview.18 The social impacts of LACV disease on families and communities who suffer the loss of a child are more difficult to identify and quantify.
Several previous publications have reported epidemiologic summaries of LACV disease; however, none have focused exclusively on LACVND in North Carolina.5,6,19,20 Therefore, the objectives of this study were to: 1) provide a descriptive epidemiological overview of neuroinvasive LACV disease in North Carolina from 2000–2020, 2) summarize clinical manifestations of LACVND among hospitalized individuals, 3) and provide updated public health recommendations based on these findings.
Methods
Deidentified case data were obtained from the North Carolina Electronic Disease Surveillance System (NCEDSS) through an IRB-approved data use agreement. Cases were classified as confirmed or probable LACVND based on the case definition criteria when the case was reported.21 The current (2015) case definition for neuroinvasive arboviral (California serogroup) disease is defined clinically by the presence of documented meningitis, encephalitis, acute flaccid paralysis, or other acute signs of central or peripheral neurologic dysfunction, and the absence of a more likely clinical explanation. The laboratory criteria for a confirmed case must have met the clinical criteria and one or more of the following: 1) isolation of the virus from, or demonstration of specific viral antigen or nucleic acid in, tissue, blood, cerebral spinal fluid (CSF), or other body fluid; 2) or 4-fold or greater change in virus-specific quantitative antibody titers in paired sera; 3) or virus-specific IgM antibodies in serum with confirmatory virus-specific neutralizing antibodies in the same or later specimen; 4) or virus-specific IgM antibodies in CSF, with or without reported pleocytosis, and a negative result for other IgM antibodies in CSF for arboviruses endemic to the region where exposure occurred. A probable case must have met the clinical criteria and have had virus-specific IgM antibodies in serum or CSF with no other testing (i.e., other endemic arboviruses).
Extracted data included classification (confirmed or probable), age, sex, race/ethnicity, date of illness onset, hospitalization, clinical signs, symptoms, and syndromes, mortality, and county of residence. We stratified age data in accordance with Erikson’s Stages of Psychosocial Development.22 Data prior to 2008 were previously imported into NCEDSS and did not contain clinical data (e.g., signs, symptoms, and syndromes). We analyzed categorical data as counts and described continuous data as median, mean, and range values. We obtained data in an Excel for Office (Microsoft Corporation) file, and reviewed each data row to remove data duplicates, assess formatting, and ensure reasonability. Missing data were excluded from the denominator during analyses. Incidence data were estimated using midpoint (2010) population counts.23 Data were analyzed using R version 4.1.1 in RStudio version 2021.9.1.372. Data were mapped according to county of residence usingArcGIS (ESRI, Redlands, CA).
Results
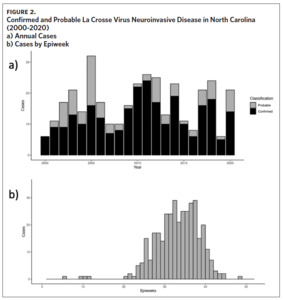
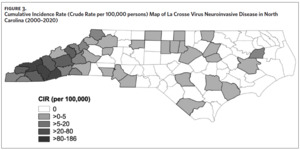
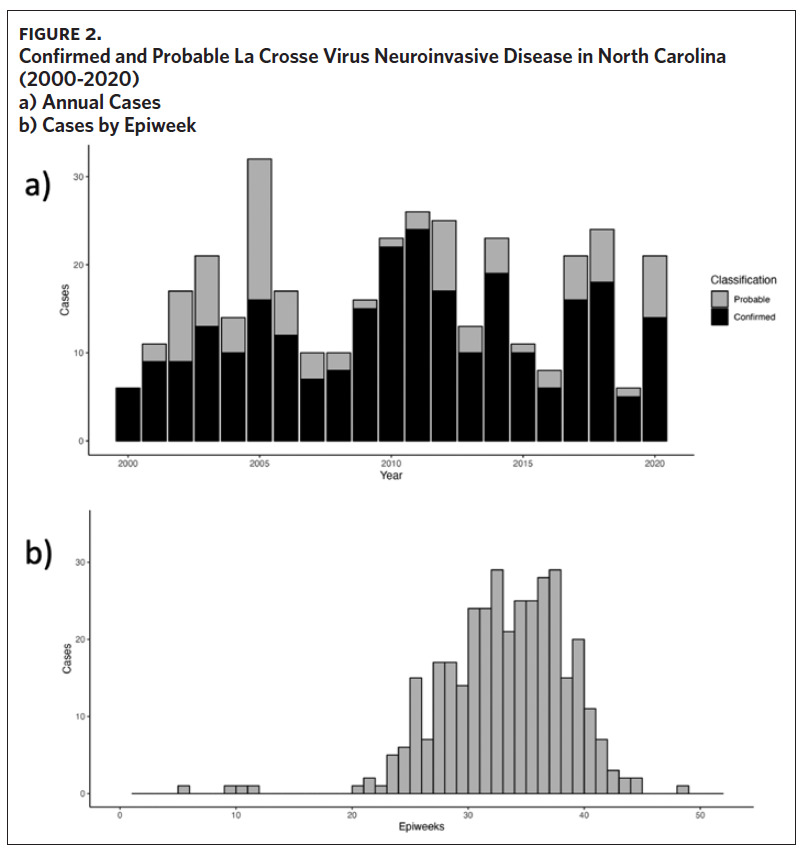
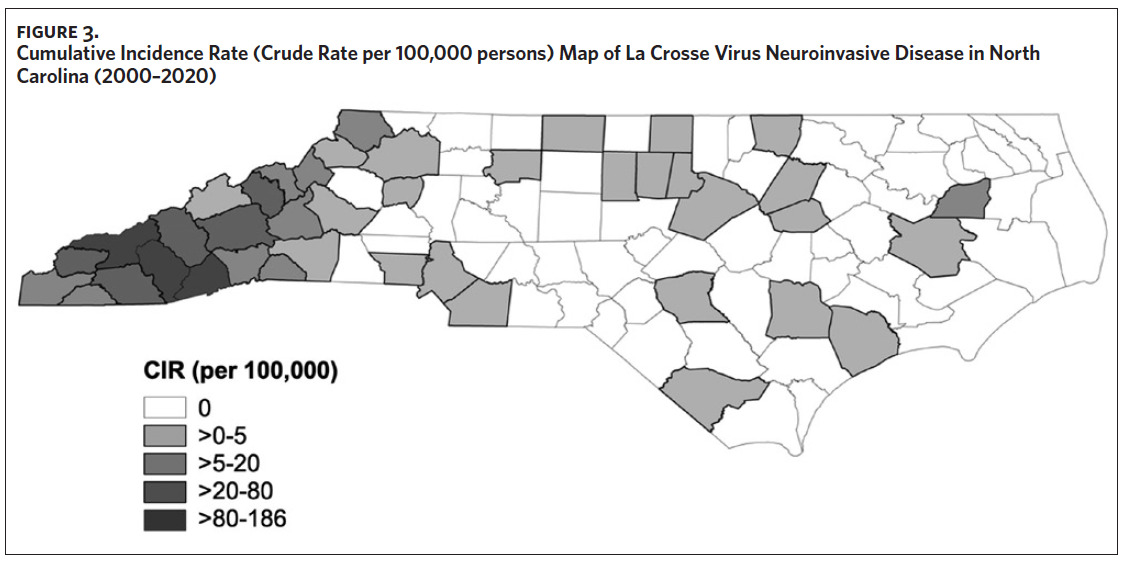
From 2000 to 2020, 355 cases of LACVND were reported in North Carolina residents, averaging 17 cases annually (Figure 2a). The majority (74.9%, n = 266) of reported cases met the surveillance case definition for confirmed arboviral (California serogroup) neuroinvasive disease. Reported cases were highest in 2005 with 32 cases and lowest in 2000 and 2016 with six cases each year (Figure 2a); no statistically significant trend (i.e., increase or decrease) was observed from 2000 to 2020 (Mann-Kendall, tau = 0.068, P-value = .67). The temporal distribution of disease onset was highly seasonal (Figure 2b) with more than 94% of cases occurring between late June and early October (epiweeks 25–41). The median epiweek of disease onset was 34 (late August) and cases were reported from week six (February) to week 49 (December). From 2000 to 2009, the median epiweek was 35, and from 2010 to 2020, the median epiweek was 33.5; the difference in median epiweek was statistically different (Wilcoxon Rank Sum Test, W = 18,097, P-value = .015). LACVND was reported in 41 of 100 counties in the state. The vast majority (92%) of cases occurred in 19 Western North Carolina counties. The largest number of cases were reported in the following counties: Buncombe (n = 98), Jackson (n = 49), Transylvania (n = 48), Haywood (n = 42), Swain (n = 26), Henderson (n = 19), and Macon (n = 10). Within these 7 counties, the cumulative incidence rate ranged from 186 (Swain County) to 18 (Henderson County) per 100,000 population (Figure 3).
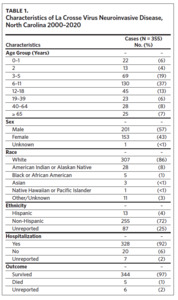
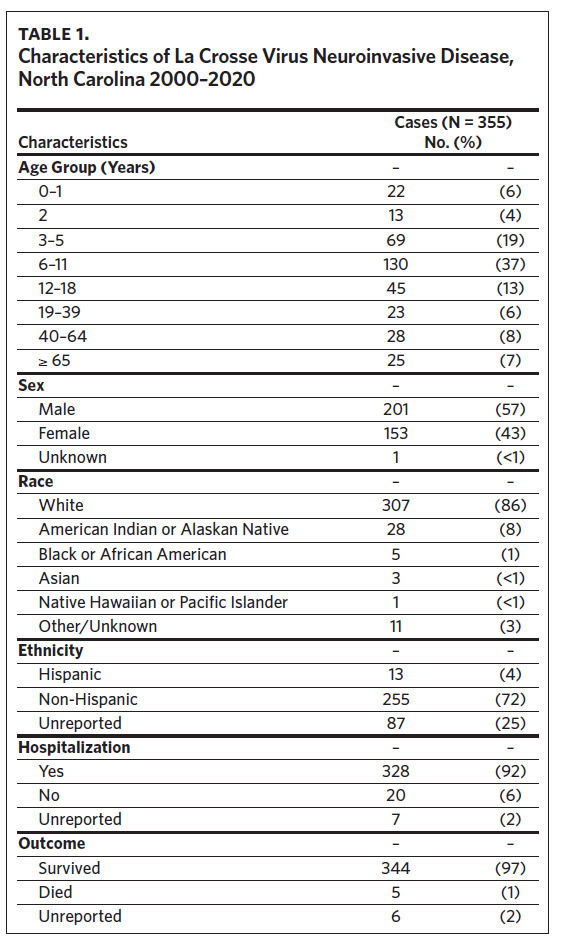
LACVND was more commonly reported in males (sex ratio: 1.3), and 79% of cases occurred in persons aged ≤ 18 years (Table 1). The median case patient age was 9 years (range: < 1–95 years). Of the 345 cases with known race, 307 (89%) were White and 28 (8%) were American Indian or Alaskan Native. Of the 348 cases with known hospitalization status, 328 (92%) were hospitalized, 94% of whom required hospitalization for more than 24 hours. Of the 349 cases with a known survival outcome, there were five deaths, with a case fatality rate (CFR) of 1.4%. The CFR was 1.5% for males and 1.3% for females. Age-group-specific CFRs were 2.3% for cases aged 6–11 years and 8.0% for cases older than age 65 years; however, these rates were not statistically different (P = .391). No deaths were reported in other age groups during the study interval.
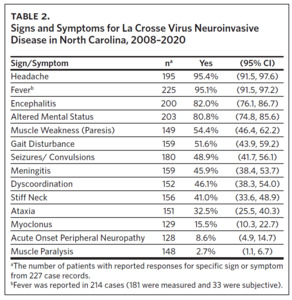
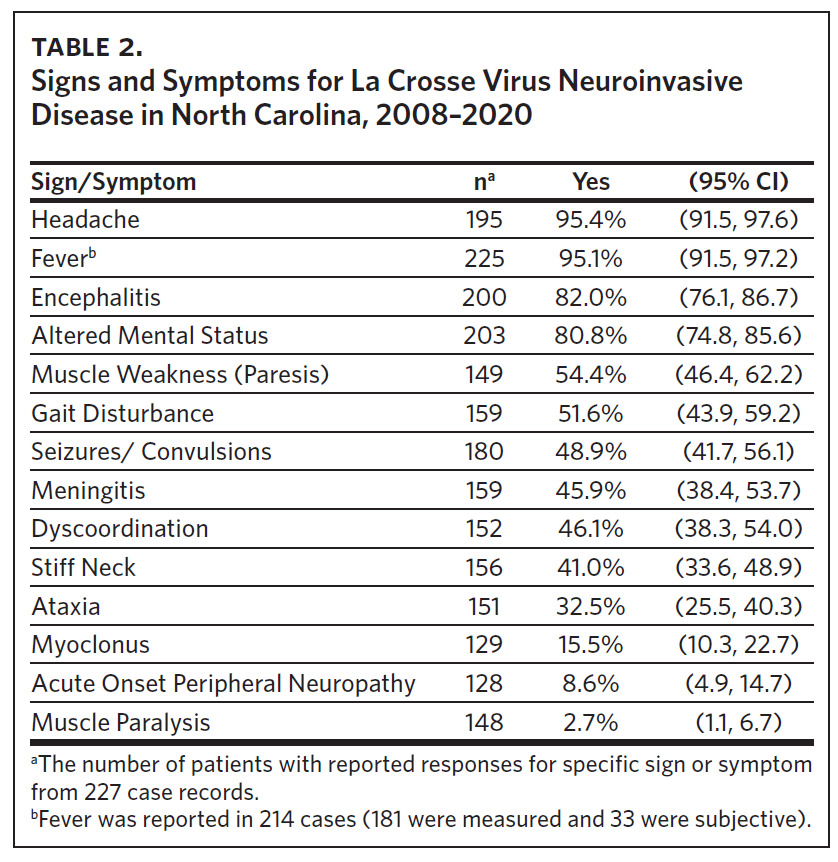
Clinical data were obtained for 227 cases occurring between 2008 and 2020 (Table 2). Fever (95%), headache (95%), and altered mental status (81%) were very common among cases. Muscle weakness (paresis), gait disturbance, seizures, dyscoordination, and stiff neck were commonly reported. Encephalitis was more common in children (87%; 95% CI: 81%–92%) than adults (62%; 95% CI: 47%–75%) (P < .001). Similarly, seizures were more common in children (54%; 95% CI: 46%–62%) than adults (27%; 95% CI: 16%–44%) (P < .01).
Discussion
This study represents the most comprehensive analysis of neuroinvasive LACV disease in North Carolina. Previous studies in North Carolina were either embedded within national data or limited to smaller case series.5,6,10,24 Our findings reinforce the importance of LACV as a regionally persistent mosquito-borne pathogen resulting in morbidity and mortality that is predominantly borne by children in Western North Carolina. Interestingly, a single age cohort (aged 6–11 years) accounted for 37% of all North Carolina cases and all three fatal pediatric cases. Although age-specific risk factors are not well described for LACV disease, behavioral factors (e.g., time outdoors, lack of personal protection measures) and biological factors (e.g., lack of acquired immunity, physiological aging), may increase exposure or severe disease risk. Even within the larger Western North Carolina region, the cumulative incidence rates (risk) of neuroinvasive LACV disease varied greatly across neighboring counties (Figure 3).
Although neuroinvasive LACV disease is considered primarily a pediatric illness, more than 20% of cases were identified in the adult population. We also identified significantly lower rates of reported seizures in adults compared to children upon presentation or hospital admission; the reported rate of seizures in children (54%) was twice as high as adults (27%). A case series report of 10 adults with neuroinvasive LACV disease in West Virginia also reported a lower, yet not statistically significant, rate of seizure occurrence (20%) in adults when compared to children (46%).25 In our study, adult cases were also less likely to have documented encephalitis (62%) when compared to children (87%). Thus, health care providers should be aware of these differences and strongly consider neuroinvasive LACV disease in the differential diagnosis of adults with fever, headache, weakness, or seizures, and corresponding residential or travel risk in endemic areas.26
Most (94%) of neuroinvasive LACV disease cases had dates of symptom onset during late July to early October (epiweeks 25–41); however, cases were reported as early as February and as late as December, which are well outside the traditional time frame of endemic mosquito-borne pathogen transmission in North Carolina. The median epiweek (33.5) from 2010 to 2020 was earlier than the median epiweek from 2000 to 2009 (35). Additional study, including extending entomological (mosquito) surveillance efforts, will be required to further assess if these cases and the shift in median epiweek represent the changing epidemiology of LACVND in North Carolina.
The public health implications of our findings are notable. The geographic persistence of LACVND in North Carolina suggests that public health interventions, particularly increased community awareness and prevention campaigns, should be targeted regionally—specifically regions 1 and 2 of the North Carolina Association of Local Health Departments. A recent study by Day and colleagues (2023), demonstrated that a high-risk cluster of neuroinvasive LACV disease persisted during 2003–2021 in seven North Carolina counties (Buncombe, Haywood, Henderson, Jackson, Macon, Swain, and Transylvania).7 Thus, these counties should be prioritized for public health and mosquito abatement programs. Similarly, children aged 3–11 years accounted for more than 50% of cases, suggesting public health interventions should focus on preschool and elementary school-aged children, their parents/guardians, and educators.
The recognition of neuroinvasive LACV disease during months not historically considered high-risk for mosquito-borne pathogen transmission suggests that entomologic (mosquito) surveillance efforts should be expanded. Although the primary vector responsible for LACV transmission remains the eastern tree-hole mosquito (Ae. triseriatus), two invasive mosquito species (Ae. albopictus and Ae. japonicus) are commonly found in LACV endemic regions of Western North Carolina.27,28 Both invasive mosquito species are capable of transmitting LACV, and Ae. japonicus is established as a temperate mosquito species, often active during cooler months.11,29,30 Additional research and surveillance are required to incriminate additional species as epidemiologically important vectors.
This surveillance summary provides evidence of persistent LACV transmission risk to humans, predominately children, in Western North Carolina. Additional evidence suggests that in some cases, risk may persist at the residential level (even specific houses) over time.10 Thus, siblings who share a residence with a LACVND case may be at higher risk for disease than other children, even in following years. Taken together, the work presented here, in context with other recent studies, suggests that public authorities should commit to a “prevent, detect, respond” approach to neuroinvasive LACV disease.31 This approach involves the following: 1) prevent: public health and the medical communities should increase public awareness of LACV disease in high-risk areas and promote effective personal protection and risk-reduction measures; 2) detect: effective epidemiologic surveillance and timely case-reporting frameworks must be enhanced within endemic regions. Likewise, neuroinvasive LACV disease should be considered in any persons (adult or child) presenting with compatible signs and symptoms with a relevant travel history to an endemic area of LACV transmission; 3) and response: public health authorities should promote risk-reduction measures (e.g., mosquito abatement, source reduction, and personal protection measures) at the residence and neighboring residences of any LACVND case32; this is especially warranted if sibling children remain present at the residence as transmission risk may persist.10
Our study has several strengths, namely that it includes more than two decades of epidemiological surveillance data, the majority of which is from a hyperendemic region of Western North Carolina. However, it also has some important limitations, including changes in NCDHHS reporting requirements during the study interval, instances of missing outcome data due to lack of patient follow-up, the potential for differences in reporting from multiple sources, and the retrospective nature of the study design. Changes to the standard case definition in 2011 required documentation of an objective fever, defined as ≥ 100.4°F (38°C). In 2014, this was subsequently changed to a less restrictive criterion not requiring the documentation of fever.21 Approximately 15% of LACVND cases in this study reported subjective fevers (Table 2), suggesting that during the 2012–2015 period, fewer cases were reported than would be expected if the current case definition had been in effect. Some analyses were limited in sample size due to missing data. Additionally, our data were restricted to North Carolina residents only, meaning that these data do not include any non-resident exposures that occurred in North Carolina. Despite these limitations, the data presented here reinforce La Crosse virus as a persistent cause of arboviral disease in children in the western region of North Carolina. Additional investment in public health capacity and biomedical research is clearly warranted.
Conclusion
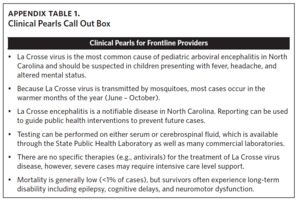
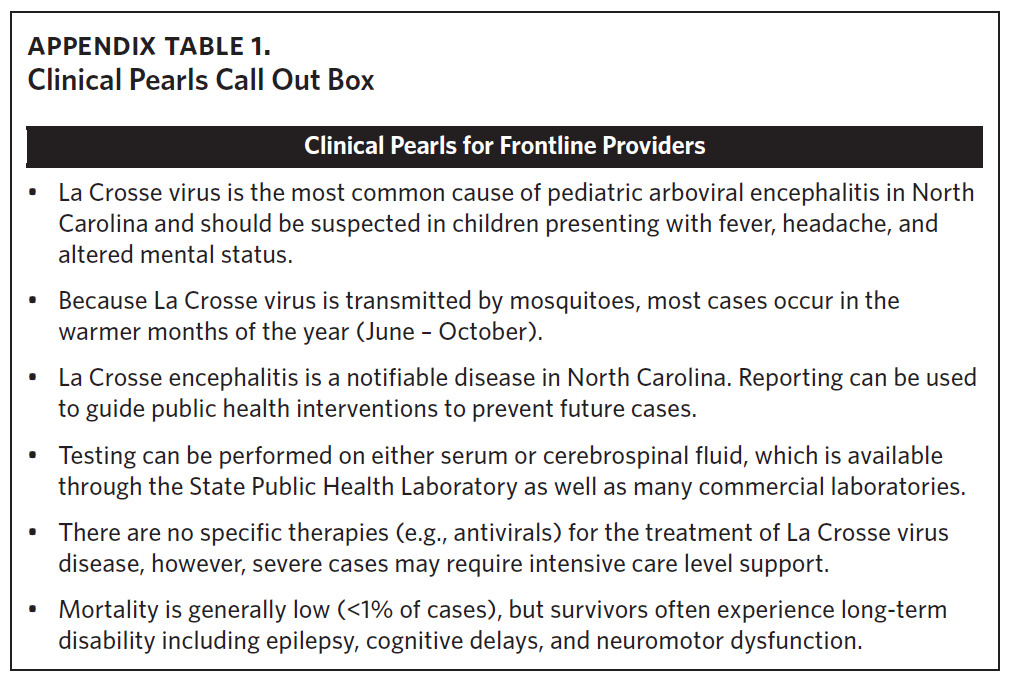
Neuroinvasive La Crosse virus disease remains endemic in Western North Carolina. Thus, clinicians and public health providers should consider La Crosse virus disease in individuals, particularly children, showing compatible symptoms and a travel history to endemic counties (See Appendix Table 1, Clinical Pearls). Our study documents La Crosse virus disease in North Carolina residents outside of historical transmission periods indicating the need for heightened clinical vigilance and improved public health surveillance efforts beyond the summer and early fall months. Taken together, the persistence of LACVND in rural Appalachian foci, combined with the lack of available vaccines or effective therapeutics, clearly classifies the disease as neglected. Public health messaging should be prioritized for both caregivers and children in Western North Carolina counties, particularly during periods of elevated transmission risk. There remains a critical need for a comprehensive strategy to prevent, detect, and respond to cases of LACVND in western North Carolina.
Acknowledgments
The authors would like to thank Mary Nordgulen (Western Carolina University Mosquito and Vector-borne Disease Laboratory) for her assistance with Figure 1. Figure 1 was created using Biorender.com (Agreement Number: OC260BN5WH). This study was supported, in part, by the North Carolina Department of Health and Human Services, Communicable Disease Branch (Contract # 43152) as an Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (NU50CK000530) subcontract (Centers for Disease Control and Prevention).
Disclosure of interests
The authors have no relevant conflicts of interest relating to this study.