Introduction
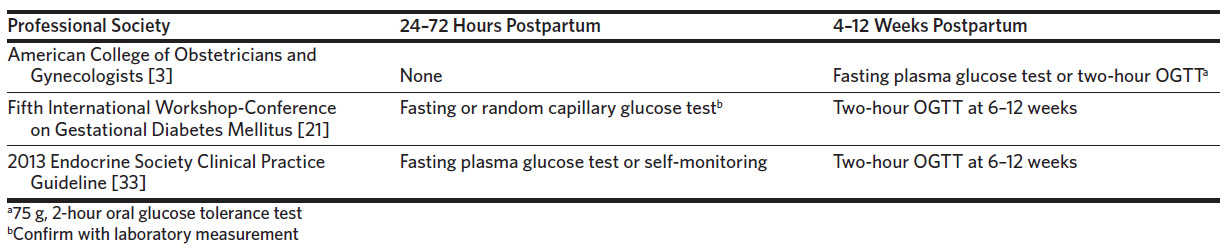
Gestational diabetes mellitus (GDM) affects 4%–12% of pregnancies in the United States, disproportionally affecting Hispanics, African Americans, Native Americans, and Asians or Pacific Islanders, and accounting for 8 in 10 cases of diabetes in pregnancy.1–3 While most cases of GDM resolve after delivery, up to 30% of women will have diabetes or impaired glucose metabolism postnatally, and patients with a history of GDM will have a 7-fold increased risk for developing type 2 diabetes mellitus (T2DM) later in life.3,4 Professional guidelines recommend postpartum screening for glucose abnormalities in women with gestational diabetes within the first 72 hours postpartum and/or at 4–12 weeks postpartum with a 2-hour oral glucose tolerance test (gold standard) or a fasting plasma glucose (FPG) test (Table 1). The American College of Obstetricians and Gynecologists (ACOG) recommends that women with gestational diabetes undergo postpartum screening for diabetes or impaired glucose metabolism with a FPG test or 2-hour oral glucose tolerance test (2-hour OGTT) and be referred to primary care.3,5 To reduce the risk of progression to diabetes, the first-line treatments for glucose intolerance are lifestyle modification with or without pharmacologic intervention.3
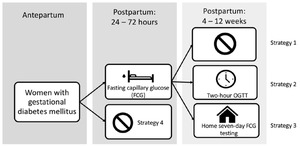
On average, approximately 40% of women do not complete a postpartum visit and more than half of women with GDM do not complete a postpartum 2-hour OGTT.6–8 Our academic institution implemented a custom protocol to improve screening of postnatal glucose intolerance. Women with GDM are screened for ongoing overt hyperglycemia with a fasting capillary blood glucose level at 24–48 hours postpartum. If positive, no further screening is performed. If negative, patients have the option to complete either a 2-hour OGTT or check their fasting glucose at home for their postpartum visit at 4–6 weeks. Regardless of the result, all women are referred to primary care. Our institution’s fasting capillary blood glucose testing immediately postpartum provides an opportunity to detect overt disease in women who otherwise would not undergo any screening because they are unable to present to their postpartum visit.
The comparative cost-effectiveness of various screening protocols for postpartum diabetes and impaired glucose tolerance at 4–12 weeks postpartum is unknown. The purpose of this study was to compare the cost and effectiveness of 4 different postpartum glucose intolerance screening strategies for women with gestational diabetes from the perspective of an OB/GYN health system at a single academic institution. We also evaluated variables that influence the effectiveness
Methods
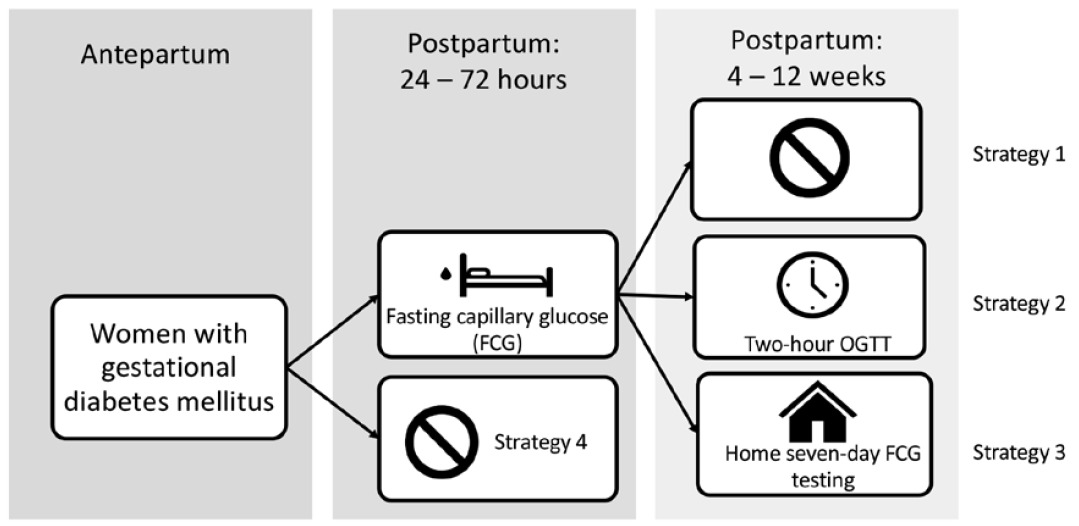
We constructed a decision model using TreeAge Pro Healthcare9 to compare the cost and effectiveness of 4 screening strategies to detect diabetes and prediabetes in postpartum women who had GDM in the index pregnancy: Fasting capillary glucose test (FCG) 24–72 hours postpartum (inpatient FCG) (strategy 1); Inpatient FCG plus a 2-hour OGTT at 4–12 weeks postpartum (strategy 2); Inpatient FCG plus home fasting capillary glucose monitoring for 7 days at 4–12 weeks postpartum (strategy 3); No screening (strategy 4). We used the software to create a simple decision model (Figure 1) to estimate the cost of each strategy by multiplying the costs associated with each screening method by the sensitivity and specificity of each screening modality and the probability of occurrence of each method. We derived clinical probabilities from the published literature and from institutional data. Our primary outcome was the per-patient cost and effectiveness of detecting diabetes and prediabetes and the incremental cost-effectiveness ratio (ICER) between strategies. The ICER is the extra cost per extra patient with diabetes or prediabetes identified. Effectiveness was the proportion of patients with disease detected and was derived from disease prevalence, sensitivity of each screening modality, and probability of occurrence of each screening modality. Sensitivity analyses were performed to examine parameter uncertainty, variance, and effect on primary outcome. The Duke Institutional Review Board provided exemption for this study.
Our model has 6 key assumptions: 1) The proportion of patients completing an inpatient FCG test does not differ across screening methods; 2) Patients with GDM already had a glucometer at home during pregnancy so its cost is not included in the home FCG testing; 3) A patient who chose to check her fasting glucose at home and returned to her postpartum visit always brought her log so that the proportion of patients returning to a postpartum visit equals the proportion of patients completing the home fasting capillary glucose monitoring; 4) The sensitivity and specificity derived from the literature for fasting plasma glucose is the same as for fasting capillary glucose; 5) The sensitivity and specificity of a FCG test is the same whether performed at the hospital or at home; 6) Two home fasting capillary glucose values that are abnormal on separate days constitute a positive screen.10
We performed a PubMed search using the search terms “gestational diabetes”[MeSH]; “diabetes” or “type 2 diabetes” or “pre-diabetes”; “costs and cost analysis”[MeSH]; “economics, hospital”[MeSH]; “oral glucose tolerance test” or “OGTT”; “two hour” or “2 hour” and “glucose” and “tolerance test”; “fasting plasma glucose tolerance test”; “fasting” and “glucose” and “tolerance test”; “diagnosis”; “screening”; “sensitivity”; “specificity”; “prevalence”; “postpartum” or “postpartum visit” or “postpartum period”[MeSH]; “early” or “immediate”.
We derived parameter estimates from articles identified per the search above, from cited studies within those articles, and from our own institutional data. We obtained retrospective prevalence data from patients who received care at the Durham County Health Department or Duke Perinatal – Durham, were diagnosed with gestational diabetes, and delivered at Duke University Hospital or Duke Regional Hospital between January 2014 and December 2018 when the Epic electronic health record was adopted, which allowed for ease of data retrieval. ICD–9 and ICD–10 codes were used to obtain data specific to patients diagnosed with GDM (unspecified), diet-controlled GDM (A1GDM), and medication-controlled GDM (A2GDM). We used patient rather than pregnancy episode as the count to avoid counting the same patient twice as would be the case if pregnancy episode were used. The data were collected by our OB/GYN Departmental Analytics Resources Team (DAART) from the Duke Health Technology Solutions (DHTS) Analytics Center of Excellence (ACE).
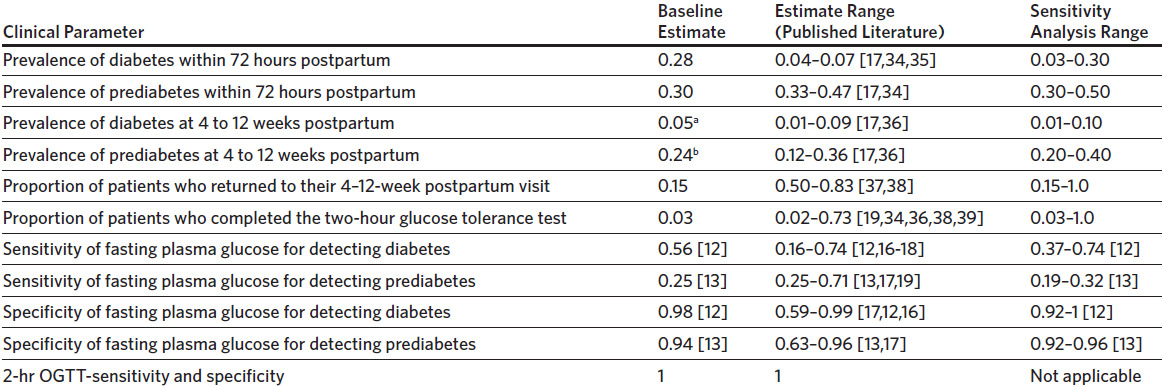
Parameters included the following: prevalence of diabetes and prediabetes among women with GDM in the index pregnancy within 72 hours of delivery, the proportion of women with GDM who return for their 4–12-week postpartum visit, the proportion of women with GDM who completed the 2-hour OGTT at 4–12 weeks postpartum, the prevalence of diabetes and prediabetes at 4–12 weeks postpartum, and the sensitivity and specificity of FPG testing for detecting prediabetes and diabetes. Since the 2-hour OGTT is the gold standard, the sensitivity and specificity are 100%. Appendix 1 shows the baseline estimates and reasonable clinical ranges for each parameter obtained for sensitivity analysis. It is important to note that the sensitivity and specificity of the FPG test reported in the literature was dependent on the FPG cutoff used to detect diabetes and prediabetes in each study.
We derived costs in 2019–2020 US dollars (Appendix 2). We obtained costs for a point-of-care (POC) capillary glucose test and the 2-hour OGTT using the 2019 Centers for Medicare & Medicaid Services (CMS) clinical laboratory fee schedule, as well as current Medicaid and other health insurance reimbursement practices at our institution. We derived costs for a 7-day supply of glucometer test strips and a 7-day supply of glucometer lancets using our institution’s outpatient pharmacy and other commercial pharmacy retail prices. The cost of detected diabetes and prediabetes was obtained from the available literature.
Clinical Estimates
We obtained the baseline prevalence of postpartum diabetes and prediabetes in women with gestational diabetes within 24–72 hours from delivery from our institutional data. Per our institutional protocol and standard guidelines in the general population, a fasting capillary glucose ≥ 126 mg/dL is suggestive of diabetes and 100–125 mg/dL is suggestive of prediabetes.11 In addition, from our institutional data we obtained the baseline proportion of patients with GDM who returned for their 6–2-week postpartum visit and the baseline proportion of patients who completed the 2-hour OGTT at 6–12 weeks postpartum. Articles identified by the literature search were examined for baseline prevalence of diabetes and prediabetes at the 6–12-week postpartum visit since we did not have this information from our institutional data.
The baseline clinical estimate for the sensitivity and specificity of FPG testing for detecting diabetes was chosen from a meta-analysis that included the cutoff of ≥ 126 mg/dL for diagnosing diabetes,12 which is the cutoff used at our institution. The sensitivity and specificity of FPG testing for detecting prediabetes was chosen from a systematic review and meta-analysis that encompassed different cutoffs published in the literature as specific data were not found for a cutoff of 100–125 mg/dL.13 Baseline clinical estimates are summarized in Appendix 1.
Cost Estimates
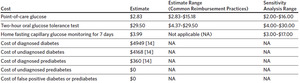
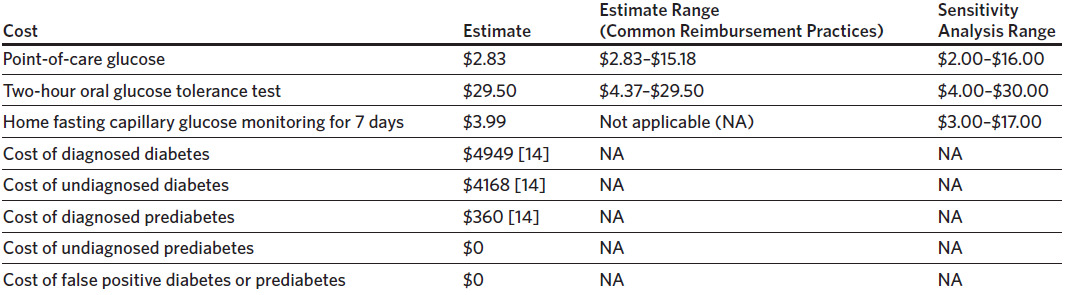
We obtained costs associated with glucose testing from current reimbursement data from various health insurances at our institution and the 2019 CMS clinical laboratory fee schedule using standard Current Procedural Terminology (CPT) codes. The baseline estimate cost for a point-of-care glucose test using CPT code 82962 is $2.83, which is the amount reimbursed by Medicaid at our institution. Seventy-five percent of patients with gestational diabetes at our institution from 2014 to 2018 used Medicaid. The baseline cost for a 2-hour oral glucose tolerance test using CPT code 82947 is $29.50, which is the amount billed to Humana Medicare Advantage at our institution. We used sensitivity analyses to account for reimbursement by other health insurance companies, as well as by CMS.
The retail value of Accu-Chek® Guide Test Strips, which are often subsidized for uninsured patients at our Duke Outpatient Pharmacy, is $0.43 per strip, or $3.01 for 7 days. Similarly, Accu-Chek® FastClix Lancets are $14.08 for 102 lancets, or $0.98 for 7 days. The cost of monitoring daily fasting capillary glucose at home for 1 week is therefore the sum of the cost of the lancets and strips, or $3.99.
We obtained estimated costs associated with diagnosed and undiagnosed prediabetes and diabetes from Dall and colleagues on the economic burden of these conditions in a single year.14 The population of interest extracted from this study was women aged < 35 years. The cost in 2012 US dollars for diagnosed diabetes was $4120, $3570 for undiagnosed diabetes, and $300 for prediabetes. These costs were converted to 2019 US dollars using the medical care component of the consumer price index15 and are shown in Appendix 2.
Sensitivity Analysis
We performed sensitivity analysis on the prevalence of diabetes and prediabetes in the immediate (within 72 hours postpartum) and 6–12-week postpartum period, the proportion of patients with GDM who return for their 6–12-week postpartum visit, the proportion of patients with GDM who completed the 2-hour OGTT, and the sensitivity and specificity of FPG testing for detecting diabetes and prediabetes. Ranges for the sensitivity analysis were chosen by the authors to best encompass the ranges published in the literature. The ranges for the sensitivity and specificity of the FPG test for detecting diabetes and prediabetes were chosen from the 95% confidence interval included in the meta-analyses from which the baseline estimates were chosen.12,13 The values were rounded up as appropriate. As mentioned, these meta-analyses did not incorporate postpartum women in their population. However, when compared to other studies that did include postpartum women but that also used lower FPG cutoffs than ≥ 126 mg/dL for a positive diabetes screen, the data ranges chosen by the authors best encompass the sensitivity and specificity in the published literature for postpartum women.12,13,16–19 Clinical estimate ranges for sensitivity analysis are summarized in Appendix 1.
We also performed sensitivity analysis on the cost estimates for the point-of-care glucose test and 2-hour OGTT, and the cost associated with glucometer test strips and lancets. The sensitivity analysis for a POC glucose test and a 2-hour OGTT included common reimbursement by different health insurance companies and by the 2019 Medicare Laboratory Fee Schedule. Medicare reimburses $3.28 for a POC fasting glucose test,20 and common health insurance companies reimburse $2.99–$15.18. Medicare reimburses $4.37 and $14.30 for a 2-hour oral glucose tolerance test (CPT codes 82951 and 82947). Data were not available for reimbursement from other health insurance companies at our institution given the lack of test performance. We obtained the cost ranges for the glucometer lancet and test strips from least to most expensive from available common retail prices. The highest cost for a 7-day supply of test strips was $14 and for a 7-day supply of lancets was $2.24, which together total $16.24. Cost estimate ranges for sensitivity analysis are summarized in Appendix 2.
Results
Base Case
The most expensive and most effective strategy using the estimate baseline parameters is a FCG 24–72 hours postpartum plus home fasting capillary glucose monitoring for 7 days at 4–12 weeks postpartum (strategy 3). The cost is $1330, and compared to strategy 2, the incremental cost is $3, the incremental effectiveness is 0.00, and the ICER is $837. Very close in cost was FCG 24–72 hours postpartum plus a 4–12-week postpartum 2-hour OGTT (strategy 2). The cost is $1328, and compared to strategy 1, the incremental cost is $9, the incremental effectiveness is 0.016, and the ICER is $571. See Table 2 for a summary of results.
Clinical estimates. The baseline prevalence of diabetes within 72 hours postpartum was 28%. In sensitivity analysis, if the prevalence remains above 10%, strategy 3 remains slightly more effective and more expensive than strategy 2. Under 10%, the difference is almost nonexistent. The baseline prevalence for prediabetes within 72 hours postpartum was 30%. Even when the prevalence was increased to 50%, strategy 3 remained slightly more effective and more expensive than strategy 2. The baseline prevalence of diabetes and prediabetes at 6–12 weeks postpartum was 5% and 24%, respectively. In sensitivity analysis, regardless of the prevalence, the effectiveness and cost of each strategy remain unchanged and strategy 3 remained slightly more effective and more expensive than strategy 2.
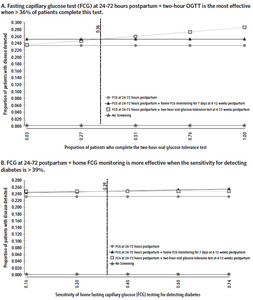
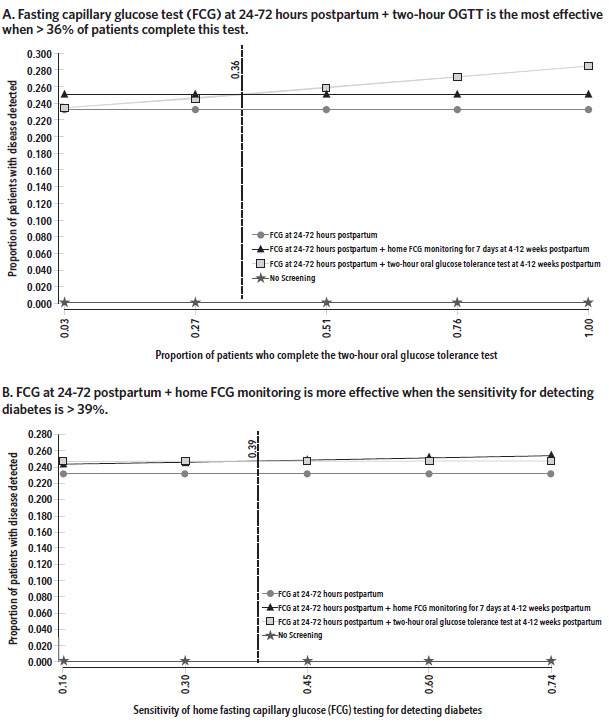
The baseline proportion of patients who returned for their 6–12-week postpartum visit was 15%. In sensitivity analysis, effectiveness and cost have a positive linear relationship with the proportion of patients who return for their postpartum visit and complete strategy 3. At 79% completion, the cost of strategy 3 is $1380 with an ICER of $624 compared to strategy 2, which cost $1328. In comparison, at 15%, the ICER for strategy 3 was $836 when compared to strategy 2. The baseline proportion of patients who completed the 2-hour OGTT was 3%. When the proportion of patients who complete the 2-hour OGTT is less than 36%, strategy 3 is more effective and more expensive than strategy 2 (Figure 2). Above this threshold, strategy 2 is linearly more effective and more expensive with rising test completion.
The baseline sensitivity for FCG for diabetes was 56%. Strategy 3 was more effective than strategy 2 when the sensitivity of home FCG testing for detecting diabetes was > 39% (Figure 3). This effect was not observed when the sensitivity of home FCG testing for detecting prediabetes improved. Variance in the sensitivity of FCG alone to detect either diabetes or prediabetes did not result in a threshold where one strategy becomes more effective than the other. Instead, strategy 3 remained more effective and also more expensive than strategies 1 and 2 regardless of the sensitivity of FCG. The baseline specificity for FPG for diabetes and prediabetes was 98% and 94%, respectively. In sensitivity analysis, no change in effectiveness or cost exists with differing specificities for FPG for diabetes and prediabetes.
Cost estimates. The baseline cost of fasting POC glucose at 24–72 hours postpartum was $2.83. As the cost increased (range $2–$16), the effectiveness of each strategy (1, 2, and 3) decreased negligibly. Increasing the cost to $16 raises the average cost per case detected by each strategy by $14 each, so that strategy 3 remained the most expensive and also most effective. The baseline cost of the 2-hour OGTT was $29.50. The average cost per case detected rose negligibly for strategy 2 with increasing cost of the test, while it remained unchanged for strategy 3; the effectiveness remained the same for each strategy regardless of the cost for this test. The baseline cost for the home fasting capillary glucose monitoring was $3.99. When varied between $3 and $17, the average cost per case detected increased negligibly for strategy 3, while the effectiveness remained the same. This variable had no effect on the other strategies. For all sensitivity analyses, FCG at 24–72 hours postpartum alone (strategy 1) remained less effective and less costly than either strategy 2 or 3. Table 5 summarizes the most influential variables on the cost and effectiveness of the different screening strategies.
Discussion
This study compared the effectiveness and health care cost per case of diabetes and prediabetes detected in patients with gestational diabetes mellitus in the index pregnancy using 3 different postnatal screening modalities plus no screening. FCG testing at 24–72 hours postpartum plus home FCG monitoring for 7 days (strategy 3) was the most effective and also the most expensive strategy compared to performing the 2-hour OGTT at 4–12 weeks postpartum (strategy 2) or to FCG at 24–72 hours postpartum alone (strategy 1). It costs an extra $845 for each additional patient with diabetes or prediabetes identified with strategy 3 compared to strategy 2. The absolute difference in cost and effectiveness among strategies was small. No postpartum screening for diabetes and prediabetes (strategy 4) had the lowest cost because it accounts for missed cases, and it is more expensive to treat diabetes ($4949) and prediabetes ($360) than to miss a case in the short term.14
The variables that influenced the average effectiveness of strategies 2 and 3 the most for detecting a case of diabetes or prediabetes were the proportion of patients completing the 2-hour OGTT at 4–12 weeks postpartum and the sensitivity for detecting diabetes with home FCG testing for 7 days. The 2-hour OGTT was more effective when more than 36% of patients completed this test. On the other hand, home FCG testing for 7 days was more effective when the sensitivity for detecting diabetes was more than 39%. It was also more effective than strategy 2 when the prevalence of diabetes was more than 10% and the effectiveness increased linearly with rising proportion of patients completing the test. The sensitivity of FCG at 24–72 hours postpartum did not influence the threshold at which one strategy became more effective than the other because we assumed that all who underwent screening completed this test (does not include strategy 4: No screening). Rising sensitivity would therefore affect all screening strategies equally.
Our model suggests that in a health care setting where fewer than 36% of patients complete the 2-hour OGTT, home FCG testing offers a reasonable alternative for postpartum glucose intolerance testing. While the sensitivity of the FCG is significantly less than that of the 2-hour OGTT (gold standard) for detecting diabetes and prediabetes, and the Fifth International Workshop-Conference on Gestational Diabetes Mellitus stated that a FPG test alone is not sensitive enough to screen for diabetes or prediabetes,21 the American College of Obstetricians and Gynecologists endorses a FPG test as an acceptable form of screening for postpartum glucose intolerance.3 Our model used FCG testing rather than FPG testing, but capillary glucose is an acceptable alternative to plasma glucose.22,23 In addition, our model accounts for 7 days of fasting capillary glucose at home rather than a single measurement, and we assumed that 2 abnormal values (≥ 126 mg/dL for detecting diabetes and 100–125 mg/dL for prediabetes) on separate days constitute a positive screen in line with the American Diabetes Association criteria for the diagnosis of diabetes mellitus.10,11 While we do not know the exact sensitivity of home FCG testing and observational studies have found differing sensitivity estimates for the FPG test in the postpartum period based on cutoff for diagnosis and timing of the test,16–18 we accounted for the uncertainty and variance associated with this estimate in our sensitivity analysis. It would, however, be of interest for future studies to examine the sensitivity and specificity of home FCG testing for 7 days using a randomized control trial.
Postpartum screening for glucose intolerance poses a clinical challenge. Both patient and institutional factors create barriers to screening.6,7,24,25 Barriers to care disproportionally affect minority populations, particularly Hispanic and Black women in whom gestational diabetes is most prevalent and who are more likely to forgo their postpartum visit and glucose intolerance screening.26,27 Underdiagnosis and lack of follow-up can lead to adverse cardiovascular, metabolic, and diabetic long-term health outcomes.28,29 The rate of screening for diabetes in women with a history of gestational diabetes by 4 months postpartum continues to remain at an average of about 50% over the last decade.26 Several studies have explored ways to improve adherence to postpartum screening, though no single strategy has been found to be the most effective.24,30,31 A strategy involving a less sensitive test, such as home FCG testing, that a greater proportion of patients are willing to complete will detect more patients with diabetes than a strategy with a test of perfect sensitivity but that only few people complete. In addition, with the advancement of telehealth, through which patients can receive follow-up remotely from home, home FCG testing offers the opportunity to decrease the number of missed diagnoses of diabetes at the 4–12-week postpartum period even if patients do return for their postpartum visit, thus resulting in improved access to care. Future studies assessing patient preferences for the 2-hour OGTT versus home FCG testing for 7 days will provide further insight into developing a screening strategy that meets the needs of the patient population.
Our study had many strengths. To our knowledge, this is the first model that compares the cost and effectiveness of postpartum glucose intolerance screening strategies in the immediate 4–12-week postpartum screening. A study by Kim and coauthors looked at screening for glucose
intolerance beyond the 4–12-week period with a FPG test, the 2-hour OGTT, or Hgb A1c to estimate the cost per case detected and cost per woman screened, percent of cases detected, and time elapsed with undiagnosed diabetes or prediabetes.32 They found that the 2-hr OGTT resulted in lower cost per case detected compared to FPG or A1c when screening every 3 years, but FPG resulted in lower cost per case detected when screening annually. Our model incorporated relevant clinical data based on our institutional experience. The estimates obtained through our model reflect the outcome of our standard practice and therefore are immediately applicable to our institution. However, they are also customizable and parameters can be modified to address the needs of other institutions’ populations. We used extensive sensitivity analysis to explore variance in data and address inaccuracies and biases of our estimates.
Our model also had certain limitations. First, while we used different sensitivity and specificity of FCG testing for detecting diabetes and prediabetes, the model was not built to evaluate the effectiveness of each strategy for detecting these 2 diseases separately. However, since our baseline FCG sensitivity for detecting diabetes was 56% and home FCG testing was estimated to be more effective at a sensitivity > 39% for detecting diabetes, then home FCG may be more effective at detecting diabetes rather than prediabetes. Next, we did not account for the costs of false positive results from our model perspective, as it was limited to the perspective of a single OB/GYN department. As patients are referred out of the OB/GYN system into the broader primary care system for follow-up of diabetes and prediabetes, the individual OB/GYN department would not incur the costs associated with a false positive result. However, this limits the model’s generalizability to the broader health system perspective. Finally, our model underestimated the cost of a missed case by limiting the horizon to 1 year. The available data used for the cost of diagnosed and undiagnosed diabetes and prediabetes came from a cost study by Dall and coauthors, that focused on a single year’s estimates.14 Limiting the time horizon to 1 year was appropriate for our model, which focused on screening in the first 3 months postpartum. However, if viewed from the perspective of the broader US health system, it would be more appropriate for the generalizability of the model to include lifetime costs for missed diabetes and prediabetes, though this is likely to have a very small impact on the overall effectiveness of our strategies. For experimental purposes, the cost of missed diabetes was increased to an arbitrary number of $30,000 in our model, and the outcome remained the same.
In summary, our model suggests that screening for postpartum glucose intolerance with home FCG for 7 days at 4–12 weeks postpartum is a viable alternative to the 2-hour OGTT after screening for overt postpartum hyperglycemia with a FCG test at 24–72 hours postpartum in practices where adherence to the 2-hour OGTT is low. Obstetric care teams may use this model as a tool to guide the development of standard screening protocols for postpartum glucose intolerance after gestational diabetes according to their program and patient characteristics. Minimizing missed opportunities for diagnosis with improved access to care is of utmost importance. Early detection and management of diabetes and prediabetes facilitates optimizing glucose control prior to a subsequent pregnancy and overall decreases the risk of disease progression, adverse pregnancy outcomes due to uncontrolled diabetes, and downstream long-term complications to both mother and infant.
Acknowledgments
The authors would like to acknowledge Adrienne Barnosky, DO, Duke Endocrinology, Duke University Health System; Dipali Pandya, Business Intelligence Developer, Departmental Analytics Resources Team (DART), Analytics Center of Excellence (ACE), Duke Health Technology Solutions (DHTS), Duke University Health System; Gina Lombardo, BS, CPC, Private Diagnostic Clinic Revenue Manager, Department of Obstetrics and Gynecology, Duke University Medical Center; Sarah Cantrell, MLIS, Associate Director for Research and Education, Liaison to Graduate Medical Education, Duke University Medical Center Library & Archives.
Disclosure of interests
No interests were disclosed.