To the Editor—The opioid epidemic continues to devastate individuals and communities in North Carolina with more than 3500 overdose deaths in 2021.1 High rates of unemployment, low rates of insurance coverage, and difficulty accessing addiction treatment have been observed in populations with opioid use disorder (OUD).2 Opioid withdrawal management commonly occurs in inpatient or residential settings and can serve as a point of entry into treatment. Often, residential detoxification facilities do not offer FDA-approved medications for OUD (MOUD).3 Opioid “detox” alone, without subsequent outpatient treatment, results in poor outcomes following discharge.4,5 Real-world experience involving care transitions and MOUD among patients from vulnerable communities remains limited. Healthy North Carolina 2030 addresses initiatives vital to reducing drug overdose deaths including increasing the use of evidence-based medications across a variety of treatment settings.6
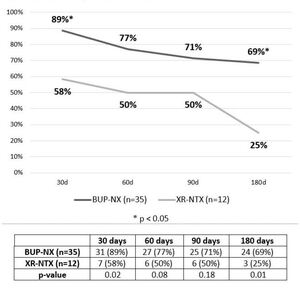
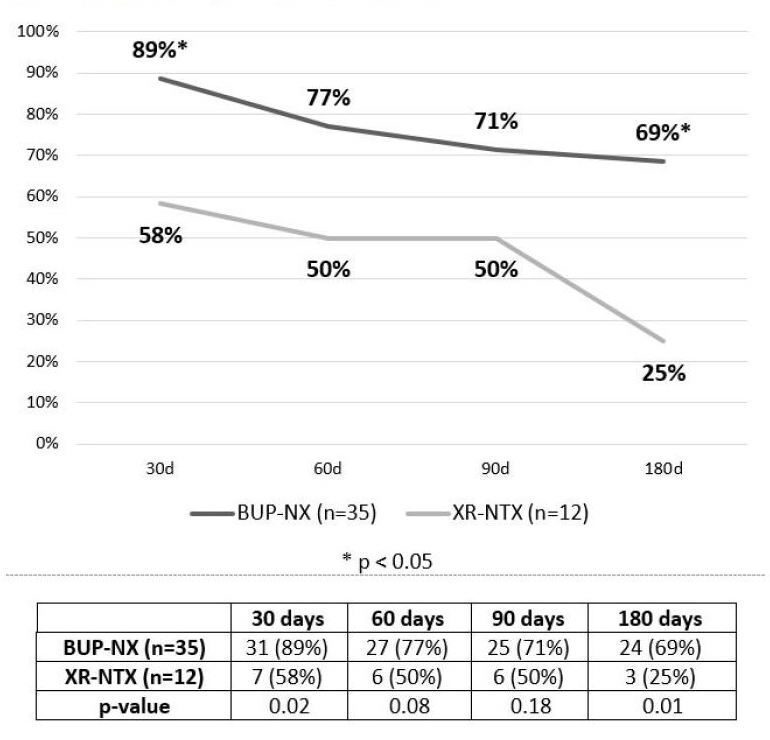
The authors conducted a retrospective chart review of 47 patients with OUD initiating MOUD, selecting either buprenorphine-naloxone (BUP-NX) or extended-release naltrexone (XR-NTX), who received care through the “detoxto- outpatient” treatment pathway between July 2018 and November 2020. Patients initiated treatment with BUP-NX or XR-NTX while admitted to an inpatient detoxification facility and were transitioned to a co-located outpatient clinic 7–14 days after discharge and followed for a minimum of six months. Methadone was not offered as the detoxification unit is not a federally licensed opioid treatment program. Approval was obtained from the Office of Human Research Ethics at the University of North Carolina at Chapel Hill. Among the 47 patients (66% male; mean age 34.7 years; 64% Caucasian; 96% uninsured), 35 initiated BUP-NX and 12 initiated XR-NTX. Patients treated with BUP-NX had higher rates of retention in outpatient treatment than patients treated with XR-NTX at 30 days (89% versus 58%, P = .02), 60 days (77% versus 50%, P = .08), 90 days (71% versus 50%, P = .18), and 180 days (69% versus 25%, P = .01) (Figure 1).
By six months, 31% of patients on BUP-NX were lost to follow-up as compared to 75% of patients on XR-NTX. One overdose death occurred in the XR-NTX group during longitudinal follow-up; no overdose deaths occurred in the BUP-NX group. See Appendix 1.
This study highlights several important aspects of the evolving opioid epidemic in North Carolina: 1) Medicaid expansion is a critical element in expanding access to evidence- based treatment; 2) although a small study, this study and others identify potential issues with XR-NTX, including challenges with initiation in the era of fentanyl7; 3) OUD treatment programs across North Carolina should incorporate the standard of care—MOUD—into treatment to mitigate the risk of opioid overdose deaths.
Author Bios
George C. Coleman, MD, MPS assistant professor, Addiction Medicine, Department of Psychiatry, University of Michigan Medicine, Ann Arbor, Michigan.
Joseph B. Williams, MD, FASAM associate professor and director of correctional psychiatry, Department of Psychiatry, University of North Carolina School of Medicine, UNC WakeBrook, Raleigh, North Carolina.
Katherine E. McDougal, MPH, PA-C clinical instructor, Department of Psychiatry, University of North Carolina School of Medicine, UNC WakeBrook, Raleigh, North Carolina.
Michael N. Zarzar, MD professor and division head, Department of Psychiatry, University of North Carolina School of Medicine, and medical director, UNC WakeBrook, Raleigh, North Carolina.
Michael H. Baca-Atlas, MD, FASAM assistant professor, Department of Psychiatry, University of North Carolina School of Medicine, UNC WakeBrook, Raleigh, North Carolina and Department of Family Medicine, University of North Carolina School of Medicine, UNC WakeBrook, Raleigh, North Carolina.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors report no potential conflicts.