Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had resulted in over 800,000 recorded deaths in the United States alone by the end of 2021.1 While many individuals with SARS-CoV-2 infection experience mild symptoms, some people experience acute, severe illness, which can result in death.2 Recent data have demonstrated that the United States has higher COVID-19 mortality rates compared with other countries, and researchers speculate that this may be partially due to the high rates of comorbidities in the United States compared to other countries.3
Studies on patients hospitalized with COVID-19 have repeatedly demonstrated an association between COVID-19 fatality and both age and gender.4–6 Studies conducted among patients hospitalized with COVID-19 have also pointed to increased death in those with comorbidities; however, there has not been full agreement on which comorbidities are most important. For example, in a study including hospital-level data from 12 states,7 odds of death from COVID-19 were highest among individuals with coronary artery disease, whereas in a study of data from over 5900 patients in a single urban medical center in New York, odds of death from COVID-19 were highest among individuals with kidney disease and second highest among individuals with dementia.8 Characteristics associated with COVID-19 fatalities in hospitalized patients may be different from those associated with SARS-CoV-2 infection-fatalities identified through statewide surveillance data.
North Carolina, with a population of 10.5 million people, has a significant rural population and a growing older adult population (17% aged 65 years or older) with 13% of residents living in poverty.9,10 The state’s most represented racial/ethnic categories include White non-Hispanic (63%), Black (22%), and Hispanic (10%).10 The North Carolina Department of Health and Human Services (NCDHHS) reports that over half (51%) of North Carolina’s population is potentially at greater risk for poor outcomes related to SARS-CoV-2 infection due to age and/or having one or more underlying health conditions.11 Although vaccinations are underway, understanding which factors are most associated with COVID-19-related death in North Carolina remains important for several reasons. First, some individuals who are at higher risk of death from COVID-19 are electing not to receive the vaccine. Second, as of this writing variants of SARS-CoV-2 continue to develop across the globe. Third, this information may aid in state-specific prevention, surveillance, and intervention strategies for future pandemics or other public health crises. Thus, using state-level surveillance data during the COVID-19 pandemic, we describe the demographics of those infected with SARS-CoV-2 and determine factors associated with infection-fatality in North Carolina residents.
Methods
Design and Setting
Our goal was to describe data, including the amount of missing data, collected as part of our state’s SARS-CoV-2 surveillance system. As a descriptive study, we did not aim to generate or test hypotheses. This retrospective cohort study used surveillance data and included all reported SARS-CoV-2-infected North Carolina residents based on specimen collection dating from March 1 to September 30, 2020 (N = 214,179). SARS-CoV-2-infected individuals are defined as those having a positive reverse-transcriptase polymerase chain reaction (PCR) or antigen test result.12
Data were provided in December 2020 and included deaths through November 2020. This descriptive assessment presents: 1) demographics and characteristics of North Carolina residents with reported SARS-CoV-2 infection; 2) SARS-CoV-2 infection-fatality rate from time of specimen collection using Kaplan-Meier curves stratified by race/ethnicity, age, sex, and the presence of comorbid health conditions; and 3) age-standardized infection-fatality rates within 30 days of specimen collection by race/ethnicity with and without comorbid conditions. This study was deemed exempt from further review by the University of North Carolina at Asheville Institutional Review Board.
Data Source and Elements
Under a data use agreement with NCDHHS, deidentified individual-level data were acquired from the North Carolina COVID-19 Surveillance System (NC COVID) that included the dates of SARS-CoV-2 test specimen collection; resident age, sex, race, ethnicity, ZIP code, and county; death attributed to COVID-19 (yes/no); date of death; and presence of 6 comorbidities: cardiovascular disease (CVD, includes congenital heart disease), chronic lung disease (includes emphysema/chronic obstructive pulmonary disease, asthma/reactive airway disease, cystic fibrosis, history of smoking, and other chronic lung diseases), diabetes, kidney disease (includes nephrotic syndrome, chronic renal failure, and other kidney diseases), immunosuppressive conditions, and metabolic disorders (inborn error of metabolism and other metabolic disorders). Our team was also able to speak with 1 health department staff member and others involved in the COVID-19 data collection effort to provide some context as to how the processes changed over time. This was helpful when considering the likely reasons for the large amount of missing data.
Statistical Analyses
Demographics and comorbidities were categorized and presented as counts and percentages of total infected individuals. As race and ethnicity measures were separately identified, we used the approach adopted by the Agency for Healthcare Research and Quality in defining a combined race/ethnicity categorization13,14: 1) Hispanic (including individuals with recorded Hispanic ethnicity equal to yes, regardless of recorded race, including missing race); 2) Black, non-Hispanic (including individuals with recorded Black or African American race and Hispanic ethnicity equal to no or unknown); 3) White, non-Hispanic (including individuals with recorded White race and Hispanic ethnicity equal to no or unknown); 4) Other non-Hispanic (individuals with race noted to be American Indian/Alaskan Native, Asian, Native Hawaiian, or “Other,” with Hispanic ethnicity marked as no or unknown); 5) missing race, non-Hispanic (including individuals where race was unknown and Hispanic ethnicity was equal to no or unknown). A more complete description of race and ethnicity categories for infected individuals is listed in Appendix 1.
The presence of each assessed comorbidity (CVD, chronic lung disease, diabetes, kidney disease, immunosuppressive conditions, and metabolic disorders) is shown as yes, no, or missing, as reported in the NC COVID system. Due to potential difficulties in clarifying the metabolic disorders category (for example, distinguishing different metabolic disorders from diabetes), analyses for metabolic disorders are shown in Appendix figures and tables only.
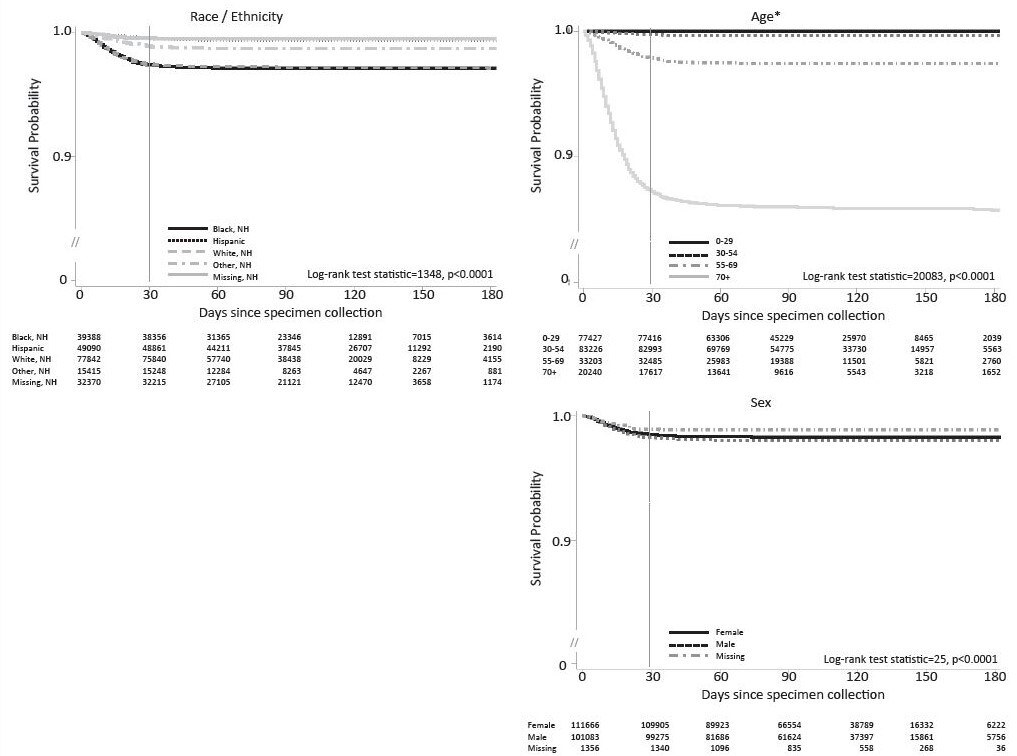
Kaplan-Meier curves illustrate the time from specimen collection of a SARS-CoV-2-positive test result to an infection-related fatality within specific strata. To ensure that the study outcome—death—occurred following SARS-CoV-2 specimen collection, infected individuals who had a date of death prior to (n = 22) or on the day of (n = 52) specimen collection were excluded from this analysis, the latter because time of test was unavailable. Individuals were censored for the time-to-event analysis on October 30, 2020 (30 days following the last included specimen collections) or at death due to a non-COVID-related cause (n = 101 individuals with death after specimen collection). The Kaplan-Meier curve graphs include the number of people at risk (the denominator) at 30-day intervals along their horizontal axis. Curves are truncated at 200 days post-specimen collection. We used a log rank test statistic for each of the Kaplan-Meier curves in order to statistically describe significant differences (at the P < .05 level) among any of the lines included in each graph.
As the majority of deaths occurred within the first 30 days following specimen collection, a dichotomous outcome variable of SARS-CoV-2 infection-fatality within 30 days of specimen collection was also created to calculate age-standardized infection-fatality. For this dichotomous outcome, in addition to excluding infected individuals with a date of death prior to or on the day of specimen collection, 67 individuals were excluded who had a non-SARS-CoV-2-associated death in the 30 days following specimen collection.
As age distributions within different race/ethnicity groups and in those with and without comorbidities were quite different, infection-fatalities were age-standardized (using 5-year age intervals through age 85+) to the full
North Carolina SARS-CoV-2-infected population used in this study. Using the North Carolina SARS-CoV-2-infected population for age standardization allows one to make estimates and draw comparisons regarding how many deaths may have been observed had all North Carolina infected individuals been Black non-Hispanic residents versus if all had been Hispanic residents versus White non-Hispanic residents. Age-standardized infection-fatalities are shown as deaths per 1000 reported SARS-CoV-2-infected individuals. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
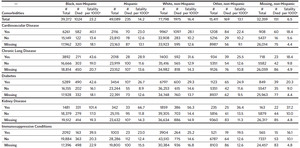
There were 214,179 total SARS-CoV-2-infected North Carolina residents captured in the NC COVID system during this study time interval (Table 1). The largest racial/ ethnic demographic was White non-Hispanic (n = 77,876, 36.4%), followed by Hispanic (n = 49,105, 22.9%), and then Black non-Hispanic (n = 39,407, 18.4%). The full race and ethnicity data from our cohort can be found in Appendix 1; notably, race was missing for 18.2% of infected individuals and Hispanic ethnicity was unknown for 30.0% of infected individuals.
The median age of all infected individuals was 38 (IQR 23–54) with 9.5% aged 70 or older and 13.4% aged 65 or older. Hispanic infected individuals had a lower median age (34, IQR 21–45) as compared to Black non-Hispanic (42, IQR 27–58), and White non-Hispanic infected individuals (43, IQR 24–61).
Cardiovascular disease was the most common comorbidity (marked as present/yes in 9.6%), followed by diabetes (8.2%) and chronic lung disease (7.0%). A high level of missing data was observed for all comorbidities ranging from 47% missing for immunosuppressive conditions to 55% missing for metabolic disorders (metabolic disorder data is in Appendix 2A). Black non-Hispanic infected individuals tended to have an overall higher prevalence of these comorbidities as compared to White non-Hispanic infected individuals at all age groups.
Kaplan-Meier curves for SARS-CoV-2-associated deaths demonstrated differences in infection-fatality over time for different race/ethnicity groups (Figure 1). Black non-Hispanic infected individuals and White non-Hispanic infected individuals showed the most infection-fatality, followed by “other race” non-Hispanic infected individuals. Infection-fatality was lowest among Hispanic individuals and for those with race data missing (P < .001). The most pronounced association was observed for age, with older age groups having higher infection-fatality. Slightly increased infection-fatality over time was observed for males.
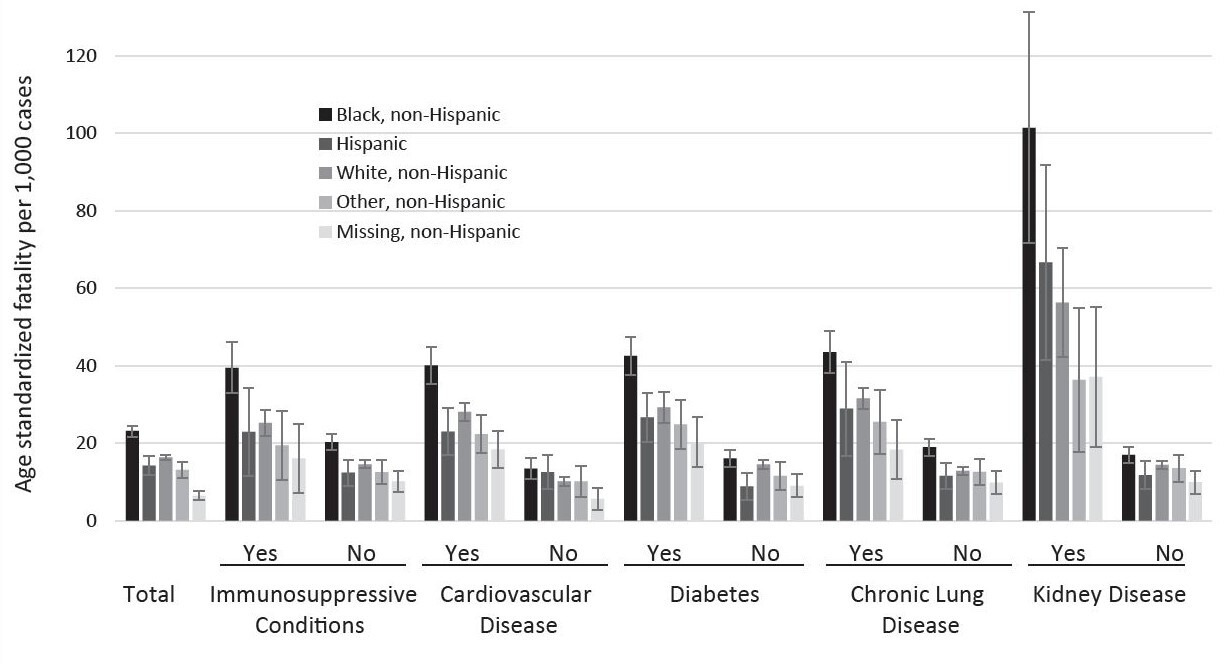
Increased SARS-CoV-2 infection-fatality was found for those having any of the comorbidities assessed, with those noted to have kidney disease showing the highest infection-fatality (Figure 2).
Age-standardized SARS-CoV-2 infection-fatalities 30 days from specimen collection were highest overall for Black non-Hispanic individuals at 23.2 (95% CI, 21.7–24.6) deaths per 1000 SARS-CoV-2-positive individuals (Figure 3 and Appendix 3). The second-highest rates were observed in White non-Hispanic individuals at 16.4 (95% CI, 15.6–17.1) deaths per 1000 SARS-CoV-2-positive individuals, followed by Hispanic individuals (14.2 per 1000; 95% CI, 11.8–16.7), “other race,” non-Hispanic individuals (13.1 per 1000; 95% CI, 11.1–15.1), and “missing race,” non-Hispanic individuals (6.5 per 1000; 95% CI, 5.5–7.6). For all race/ethnicity groups, kidney disease showed the highest age-standardized infection-fatality ranging from 101.4 deaths (95% CI, 71.6–131.2) per 1000 Black non-Hispanic SARS-CoV-2 positive individuals to 36.4 deaths (95% CI, 17.7–55.0) per 1000 for “other” non-Hispanic individuals. The presence of any of the comorbidities was associated with a higher age-standardized infection-fatality rate as compared to not having the condition or having information missing for the condition. Black non-Hispanic infected individuals had the highest age-standardized infection-fatality rate for each of the assessed comorbidities.
Discussion
This study describes demographics, the presence of comorbidities, and infection-fatality among North Carolina residents identified as infected with SARS-CoV-2 via the state’s surveillance efforts. Numerous other studies that have analyzed COVID-19 death in hospitalized patients4–6,15–17 demonstrate that age, and less so sex, was associated with increased SARS-CoV-2 infection-fatality.
Results from the current study demonstrate several key findings related to race and ethnicity. First, as has been demonstrated in a county-level surveillance assessment in Fulton County, Georgia, this study found higher age-standardized SARS-CoV-2 infection-fatality in Black non-Hispanic infected individuals as compared to White non-Hispanic and Hispanic infected individuals, both overall and with the noted presence of comorbidities.18 Additionally, data from this study indicate that the presence of comorbidities differed by race and ethnicity, with Black non-Hispanic and Hispanic infected individuals having higher proportions of comorbidities across all age groups. These findings were noted in other studies as well,8,19 highlighting longstanding health inequities; Black non-Hispanic infected individuals were more likely to have comorbidities and showed higher infection-fatality risk with comorbidities.20
Lower SARS-CoV-2 infection-fatality was found in this study for Hispanic infected individuals as compared to White non-Hispanic infected individuals. This may be attributed to differences in testing strategies among specific groups that focused on outbreaks at workplaces or residential settings as the pandemic progressed in North Carolina. Lower infection-fatality among infected Hispanic individuals was also observed in a study where authors used county-level SARS-CoV-2 data from Fulton County, Georgia.18 While our study found lower SARS-CoV-2 infection-fatality among infected Hispanic individuals, other studies assessing COVID-19 mortality have overwhelmingly found higher mortality due to COVID-19 in Hispanic populations.21–23 The findings present in other studies may be driven in part by the higher infection rates per population due to social and structural vulnerabilities, including the need to continue working in occupations with higher risks of SARS-CoV-2 exposure throughout the pandemic.24
When comparing the race/ethnicity of SARS-CoV-2 infections to the racial makeup of the state population, our sample of the Hispanic population has a disproportionately high number of individuals with reported SARS-CoV-2 infections, followed by the Black non-Hispanic population, as compared to the White non-Hispanic population. Additionally, data from our study indicate differences in age distributions of those infected with SARS-CoV-2 by race and ethnicity. Specifically, Black non-Hispanic and Hispanic infected individuals in this sample tended to be younger than infected individuals who were White non-Hispanic. These results add to the findings by Turner and colleagues that in North Carolina, not only are Black non-Hispanic and Hispanic individuals disproportionately affected by SARS-CoV-2 infection, but younger Black non-Hispanic and Hispanic individuals are disproportionately affected.25
Age was highly correlated with SARS-CoV-2 infection-fatality in North Carolina, findings that are consistent with prior studies on COVID-19-associated death in hospitalized populations and infection-fatality identified using surveillance data.4–6,15,18
All chronic diseases analyzed were associated with increased SARS-CoV-2 infection-fatality in this North Carolina-based sample. These results coincide with prior analyses of associations of chronic diseases and COVID-19 death in both hospitalized populations8,26 and in a county-level surveillance assessment in Fulton County, Georgia.18 We found that those with kidney disease had the highest infection-fatality. Similarly, a meta-analysis examining 11 chronic conditions among 25 studies found the highest average risk ratio for death from COVID-19 among individuals with kidney disease.27 However, in another review of 41 studies, kidney disease was the third most strongly correlated with death from COVID-19, following cardiovascular and cerebrovascular disease.28 Others have likewise demonstrated greater COVID-19 fatality as the number of coexisting comorbidities increased.17 In our North Carolina sample, individuals with kidney disease were more likely to have multiple other comorbidities compared to individuals with other forms of chronic disease (data not shown). This may partly explain the larger association with infection-fatality.
Our results further demonstrate that older individuals, especially those with comorbidities, may be at greatest risk of death if infected with SARS-CoV-2. This finding may add further support to the preferential vaccination rollout efforts to protect such individuals when supplies and resources are limited. As North Carolina continues to make efforts to protect and care for people during the pandemic, older adults and those with chronic kidney disease may be of particular interest as an at-risk population based on our retrospective analysis.
Results from this study are limited by missing and incomplete data, in part due to time and resource restrictions as infections quickly increased across North Carolina and individual public health department staff members’ need to engage in this work within their own contexts and resource constraints. Thus, contacting infected individuals and connecting them with resources and support may have been prioritized above the collection of ancillary data. Infection-fatality estimates by race/ethnicity may be influenced by the sizable proportion for whom race/ethnicity was not identified in this data set. Infection-fatality estimates for those with an identified race/ethnicity may be over or underestimates, based upon the composition among those with missing race/ethnicity. Additionally, differences in reported infected individuals by race/ethnicity were likely influenced by various testing campaigns related to facility outbreaks (such as meatpacking factories, prisons, and nursing homes). This may have led to certain groups having more identified and reported asymptomatic infections in healthy workers.
Despite these limitations, a strength of the current study is its focus on state-level, surveillance-based data in North Carolina for factors associated with SARS-CoV-2 infection-fatality. Many previous studies have assessed comorbidity in hospitalized patients, excluding both healthier patients who do not present to the hospital and the sickest patients who may choose not to seek hospital-based end-of-life care. In addition, care was taken not to draw conclusions that may be misleading or inappropriate. As such, this study represents rich, descriptive, correlational data that will be useful for practitioners and policymakers in North Carolina as they consider the next steps in the COVID-19 response as well as future pandemic planning. In addition to providing information on factors associated with SARS-CoV-2 infection-fatality, this study showed how academic and state partners can work together to use surveillance data during a pandemic.
Conclusion
Among a surveillance system-based sample of North Carolinians with SARS-CoV-2 infection, race/ethnicity, age, and comorbidities (most notably kidney disease) were associated with infection-fatality in a similar manner to studies assessing COVID-19 fatality in hospitalized patient populations in the United States. These findings suggest that in addition to age and other nonmodifiable variables, systematic differences in social conditions and opportunity may increase the risk of SARS-CoV-2 infection-fatality among Black Americans compared to other races/ethnicities. Results from this study also contribute to the growing body of evidence supporting prevention and treatment for non-White individuals for chronic and infectious diseases.
Financial support
This project was supported by a COVID-19 response grant from the North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill.
The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the North Carolina Department of Health and Human Services, Division of Public Health. Data were sourced from the NCDHHS Division of Public Health.
Disclosure of interests
Dr. Denslow, Dr. Rote, Dr. Wingert, and Dr. Sexton report no conflicts of interest or other sources of financial support. Dr. Hanchate receives funding from the NIH, HRSA, Duke Foundation, JPB Foundation, and the Kate B. Reynolds Charitable Trust. He has no conflicts of interest to report. Dr. Lanou receives funding from the Kate B. Reynolds Charitable Trust and the Administration for Community Living and does not have any conflicts of interest or other sources of financial support to report. Dr. Westreich consulted with PRI Healthcare Solutions on behalf of Sanofi-Pasteur within the last 12 months on topics unrelated to COVID-19 and does not have any conflicts of interest to report. Dr. Halladay is an academic researcher with a university faculty appointment as a Full Professor in the Department of Family Medicine at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, with current funding support from the Patient Centered Outcomes Research Institute, the National Institutes of Health (NIA and NHLBI), and the Agency for Healthcare and Quality. She does not have any conflicts of interest or other sources of financial support to report.
Author biographies
Sheri Denslow, PhD, MPH epidemiologist/statistician, Department of Research, University of North Carolina Health Sciences at Mountain Area Health Education Center, Asheville, North Carolina.
Aubri Rote, PhD associate professor and chair, Department of Health and Wellness, University of North Carolina at Asheville, Asheville, North Carolina
Jason Wingert, PhD, PT professor, Department of Health and Wellness, University of North Carolina at Asheville, Asheville, North Carolina
Amresh D. Hanchate, PhD professor, Social Sciences and Health Policy, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Amy Joy Lanou, PhD professor, Department of Health and Wellness; executive director, North Carolina Center for Health and Wellness, University of North Carolina at Asheville, Asheville, North Carolina
Daniel Westreich, PhD professor, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
Kedai Cheng, PhD assistant professor, Department of Mathematics, University of North Carolina at Asheville, Asheville, North Carolina
Laura Sexton, MPH, RDN, LDN research assistant, University of North Carolina at Asheville, Asheville, North Carolina; owner, Sage Nutrition Associates, Asheville, North Carolina.
Jacqueline R. Halladay MD, MPH professor, Department of Family Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina