State of the Problem
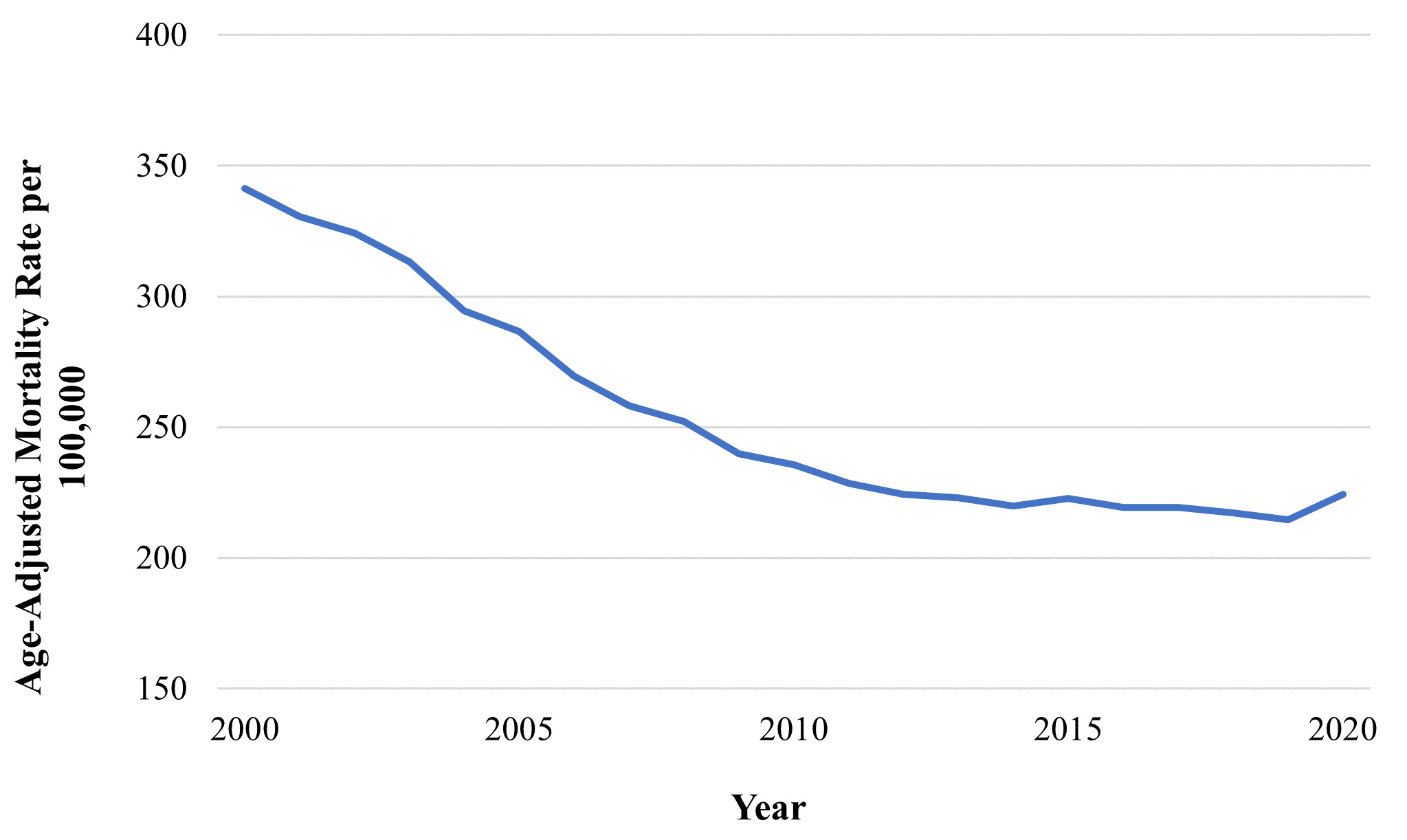
Decades-long improvements in prolonging life expectancy in the United States have stalled in recent years as promising trends in the reduction of cardiovascular disease (CVD) mortality have decelerated and, in some states, reversed.1–3 In North Carolina specifically, CVD mortality is increasing and has surpassed cancer as the leading cause of death (Figure 1).4,5 North Carolina also fares worse than the United States overall in the age-adjusted prevalence of major CVD risk factors: 36% of residents are obese, 36.8% have high cholesterol, 34.7% have hypertension, and 12.7% have diabetes.6
Further, inequality in the prevalence and mortality of CVD by race, ethnicity, education, and income is persistent across the state.4,5 Over 1 million North Carolina residents find themselves financially unable to access quality health care and the state ranks 10th in the nation for percentage of residents without health insurance.7 Lack of health insurance and quality health care are widely accepted as significant contributors to premature death.8 Screening for and treatment of major cardiovascular risk factors like hypertension and high cholesterol are also significantly lower among uninsured adults.9,10 Outcomes are particularly bleak in rural North Carolina communities, which account for nearly 40% of the state. Such areas have been identified as having some of the nation’s highest stroke morbidity and mortality.11 However, in December 2023, the North Carolina Department of Health and Human Services launched Medicaid expansion, which will increase access to health care for over 600,000 residents across the state, a promising policy development for improvement of health equity among lower-income and rural North Carolinians.
Since 2013, the Sudden Unexpected Death in North Carolina (SUDDEN) study has identified and reviewed sudden unexpected death in residents between the ages of 18 and 65 in Wake County, which includes the capital city of Raleigh and a racially and socioeconomically diverse population.12 The SUDDEN study identified that out-of-hospital sudden death had an incidence of 64 per 100,000 population in Wake County between 2013 and 2015 and was significantly higher among Black residents (107 per 100,000 population) than White residents (60 per 100,000 population). A lower household income and residence in a rural neighborhood were also independently associated with an increased risk of sudden unexpected death.13 The majority of sudden death certificates analyzed (53%) had a cardiac cause listed as the primary cause of death, and major CVD risk factors were highly prevalent: 58% of subjects were hypertensive, 43% had dyslipidemia, 33% were obese, and 31% were diabetic.14 Research efforts that focus on defining the distribution and determinants of sudden death with a cardiac cause (i.e., sudden cardiac death) are emblematic of the profound public health significance of CVD, as sudden cardiac death accounts for the greatest burden of natural death in the United States, claiming about 325,000 adult lives annually.15 Coronary artery disease is the leading pathological cause of sudden cardiac death, responsible for up to 80% of such fatalities in the United States.16 Among SUDDEN subjects, however, only 19% were diagnosed with coronary artery disease prior to their death despite 80% having visited a physician in the previous two years.17 These findings indicate a need for more timely and efficient identification of North Carolina residents living with undiagnosed coronary artery disease.
Identifying individuals at highest risk of CVD also presents a major challenge. Traditional cardiovascular risk estimates are grounded primarily in vascular risk factors such as blood pressure, cholesterol levels, and diabetes.18,19
However, risk scores derived from these risk factors may over- or underestimate risk in certain patient subgroups and lack validation in individuals under age 40.20 In 2008, the Framingham Heart Study introduced the concept of “cardiac age” to estimate 10-year CVD risk by assessing the age of the vascular system relative to chronological age.18 The intuitive concept of cardiac age is particularly useful in simplifying risk communications to patients for increased adherence to lifestyle changes and treatments.19 Regardless, the predominant and oftentimes exclusive focus on vascular risk factors may be a source of missed opportunity for the early detection of those at high CVD risk. For example, extending the concept of cardiac age to assess cardiac structure and function is a complementary strategy that may improve CVD risk prediction and classification in North Carolina and the United States. The inclusion of measures of abnormal cardiac structure and function have been shown to improve heart failure, stroke, and sudden cardiac death prediction.21–23 However, to date extending risk prediction models to include cardiac structure and function measures for population screening has been limited by availability and cost of modalities, such as echocardiography and coronary calcium imaging.
The electrocardiogram (ECG) is a cornerstone diagnostic tool for the detection of CVD given its simplicity, low cost, and scalability. It possesses the potential to expand current cardiac estimation by incorporating assessment of cardiac structure and function. ECGs record the heart’s electrical activity, yielding a wealth of valuable insights into cardiac anatomy and performance. Nevertheless, only a fraction of this information is routinely utilized to inform clinical decision-making, and a recent meta-analysis found cardiologists’ ECG interpretation accuracy ranged from 49% to 92%.24 These observations highlight a significant need to improve utilization and interpretation of ECGs to augment current risk-prediction frameworks.
Opportunities for Improvement and Innovation
The integration of artificial intelligence (AI) in health care holds considerable promise for process automation, error minimization, and novel phenotyping and prognostication, together translating into improved quality and outcomes. Broadly, AI is a field of computer science concerned with equipping systems or machines with capabilities to perform tasks that would typically require human intelligence, such as recognizing patterns, classifying data, and making predictions. In this way, AI has a unique potential to automate ECG interpretation with more precision and less bias than traditional human performance. In recent years, AI advancements have allowed a more comprehensive evaluation of a patient’s raw ECG waveform.25,26 Further, machine learning, a subset of AI in which machines learn and make decisions without being explicitly programmed to do so, possesses the potential to detect and develop new CVD risk phenotypes from biologically enriched ECG data.25,26 Developing modern characterizations of subclinical CVD beyond current risk factors will be critical for informing early preventive and interventional strategies in our communities.
The application of AI to ECG data has successfully predicted the risk of heart failure, atrial fibrillation, a variety of cardiomyopathies, and mortality, harnessing its predictive power to identify multiple novel ECG parameters that are incompletely understood.27–29 Machine learning techniques involving deep neural networks have also been used to predict cardiac age from raw ECG waveforms, which, if significantly greater than chronological age, has proven to be a powerful predictor of myocardial infarction, atrial fibrillation, left ventricular systolic dysfunction, heart failure, and mortality.30–32 This novel approach to capturing cardiac age from broad ECG data provides a window into the heart’s functional capacity and is independent of traditional ECG features and existing measures of biological age.31,32 Independence from customary ECG measures may reflect the algorithm’s ability to detect subclinical CVD that has eluded conventional diagnostic tools.32,33 The AI-enabled ECG age metric represents a novel biomarker with substantial potential for enhancing risk stratification of CVD. Such a tool may be especially valuable in North Carolina clinical practice given that North Carolina residents ranked 10th-highest in the nation for elevated cardiac age according to the Framingham criteria.34 Given the increasing burden of CVD morbidity and mortality in North Carolina, innovative, evidence-based methods such as the integration of AI into clinical screening practices provide a promising new avenue for primary prevention and risk communication.
Artificial intelligence is poised to make dramatic advancements in the near future, and one must consider potential implications and consequences for the diverse health care landscape in the United States and North Carolina. There is evidence of a delay in implementation of evidence-based best practices into rural clinical settings, where primary care facilities are often less well-funded and staffed than their more urban counterparts.35 The integration of improved, AI-enhanced risk stratification into clinical workflows in a manner that apportions health care burden from overextended patient centers in such areas offers a hitherto unavailable opportunity to tackle several of the challenges arising from health care inequity across our state and improve patient outcomes. The ECG’s well-established role in current clinical practice and relative affordability make the prospect of AI-ECG technologies especially attractive. With this said, priorities moving forward must include ensuring novel technologies are rigorously supported by clinical evidence, distributed equitably to clinical centers throughout the state, and integrated with actionable protocols that take advantage of centralized resources and expertise while meeting ethical and regulatory standards. In 2015, a team at the University of North Carolina at Chapel Hill began administering a digital risk stratification system through linkage with electronic health records in 219 small clinics (< 10 physicians) across the state, laying the groundwork for coordinated efforts to reduce the risk of cardiovascular events in our communities by augmenting tools already standard in clinical settings. By 2018, the initiative was highly successful in accelerating implementation of new evidence into rural primary care facilities and stratifying CVD risk in these patient populations.36 Such evidence highlights the adaptability and willingness of health care centers in North Carolina to incorporate innovative technologies into conventional care procedures with the aim of improving patient outcomes.
Additional points of caution prior to introducing AI into clinical practice include concerns around data quality, bias, drift, and operational transparency, among others.25,26,37 The generalizability and scope of AI algorithms rely heavily on the quality and diversity of the data from which they are trained. Thus, unbiased data representative of the North Carolina population is essential to ensuring widespread applicability and benefit. Data drift refers to changes in the statistical distribution of an AI algorithm stemming from variations in the data used for training the algorithm and data the algorithm encounters during operation. In clinical environments, data drift can emerge due to evolving patient presentations, and thus AI model monitoring and maintenance will be essential for long-term usage.37 Finally, many AI models employing deep learning, such as the ECG-derived cardiac age algorithm previously described, have an intrinsic “black-box” nature, implying an incomplete understanding of the precise data parameters guiding their decision-making processes. Achieving a more comprehensive interpretation of algorithm properties will foster provider trust in making clinical decisions based on AI-generated insights.
Conclusions
In the face of an increasing burden of CVD in North Carolina, it is essential that we continue exploring innovative solutions and cultivating collaborative efforts that transcend traditional health care boundaries to address the unique needs of our diverse communities. Medicaid expansion offers a promising policy development that may improve health equity and access to care for a substantial proportion of the state’s population. There is remaining opportunity in clinical practice to grow beyond an exclusive focus on vasculature for CVD risk assessment and optimize the rich insight into cardiac structure and function provided by biologically enriched ECG waveforms. The potential for AI to predict a wide variety of cardiovascular conditions, along with its ability to estimate cardiac age from ECGs, presents an attractive option for precision phenotyping of subclinical CVD. As we look toward the future, the integration of AI into clinical screening and risk stratification practices holds great promise for improving CVD outcomes in North Carolina.
Acknowledgments
This work was funded in part by a training grant from the National Heart, Lung and Blood Institute (T32HL129982).
Disclosure of interests
The authors disclosed no potential conflicts of interest.