In the United States, cardiovascular disease accounts for at least 25% of pregnancy-related deaths, making it the leading cause of maternal mortality.1 Individuals with preexisting cardiac disease are at higher risk of pregnancy-related cardiac complications.2 As rates of pregnancy among individuals with preexisting cardiac disease are increasing in the United States, determining optimal management of these individuals is an important component of maternal morbidity and mortality reduction.3
In both pregnant and non-pregnant individuals in the Southern United States, there are notable racial and geographic disparities in cardiovascular outcomes. Cardiovascular mortality is increasing among residents of the rural Southern United States.4 Higher age-adjusted mortality rates in individuals who live in the rural South are due to potentially preventable causes of heart disease,5,6 lower emergency department visits and hospitalizations for heart failure,7 and less access to a suitable level of care to meet the patients’ individual needs.8 There are significant disparities in pregnancy-related mortality ratios for patients in rural versus urban areas throughout the United States.9,10 Similarly, there are large disparities in obstetric outcomes between non-Hispanic Black and White individuals, both in terms of maternal mortality rates and cardiovascular complications of pregnancy. Black women have higher pregnancy-related mortality rates, morbidity rates, and cardiovascular complications of pregnancy compared to White women.11 These differences persist with education level and widen as women age.12 Specifically in North Carolina, non-Hispanic Black individuals have pronounced disparities in all causes of severe maternal morbidity (SMM) and mortality including 2.4-fold higher rates of cardiac-specific causes.13 The contribution of preexisting cardiovascular diseases to these disparities is unknown.
Our objective was to identify the volume of births with known preexisting maternal cardiac disease in North Carolina and to further evaluate the association between known maternal cardiac disease (based on modified World Health Organization categories) and outcomes. We additionally aimed to identify disparities in outcomes between urban and rural residents, and Black and White individuals in North Carolina.
Methods
This was a retrospective cohort study using the 2019 North Carolina Statewide Inpatient Database (SID) from the Healthcare Cost and Utilization Project, sponsored by the Agency for Healthcare Research and Quality.14 The SID is an administrative dataset including all inpatient admissions for non-federal (e.g., excluding military facilities), short-stay hospitals in North Carolina. Available patient data for each discharge include demographic information such as age, sex, race and ethnicity, primary payor, and individual’s home ZIP code. Race and ethnicity were combined as a single variable as reported by the hospital, which is likely a combination of self-reported and observed race and ethnicity. International Classification of Disease, 10th Edition (ICD-10), Clinical Modification diagnosis codes (with present-on-admission indicators included to denote whether the diagnosis was noted at time of hospital admission or occurred during the hospital stay) and procedure codes, hospital length of stay, discharge disposition, and delivery hospital characteristics were also provided. It is not possible to follow individuals across hospitalizations in this dataset, and thus outcomes were limited to the delivery hospitalization.
An indicator variable available in the 2019 North Carolina SID to identify delivery hospitalizations was derived by the data supplier based on diagnosis and procedure codes. This indicator variable was used to identify the cohort of interest. The exclusion criteria included individuals not residing in North Carolina, delivered before 20 weeks of gestation, or who had a missing hospital identifier. The cohort was stratified by preexisting cardiac disease. The modified World Health Organization (mWHO) classification system stratifies pregnancy risk from cardiac disease into categories ranging from no detectable increased morbidity and mortality risk to such high risk that termination of pregnancy is recommended.15 The mWHO strata typically encompass five different categories of risk: I, II, II/III, III, and IV. However, due to limitations in ICD-10 diagnosis codes, it is not possible to identify cardiac disease with sufficient granularity to assign individuals to each category (e.g., there are no billing codes for severity of lesion). Therefore, we combined categories of cardiac risk into those ranging I–II, and II/III–IV (Appendix 1). We have previously used this categorization to describe maternal cardiac disease in national administrative datasets.16 We identified other comorbid conditions using a validated comorbidity index.17 For both cardiac disease and comorbid conditions of pregnancy, we used only those diagnosis codes that were listed as present on admission or for which present-on-admission coding is not pertinent; we did this to identify conditions that were not a complication of delivery but instead were present at the time of admission.
Hospital characteristics were identified using the Medicare Provider of Services files. These included hospital ZIP code, annual delivery volume, and presence of coronary critical care units and a cardiac surgical program. ICD-10 procedure codes for all hospitalizations during the 2019 year were used to identify hospitals that could provide extracorporeal membrane oxygenation (ECMO) services and intra-aortic balloon pump procedures (IABP). We defined “cardiac hospitals” as those capable of providing ECMO, IABP, cardiac surgery, and cardiac care unit (CCU) services, since these services could be required emergently during cardiopulmonary decompensation. Individual ZIP codes were then converted into Rural-Urban Commuting Area (RUCA) codes, which are based on population density and commuting flow in selected ZIP codes. The resulting RUCA codes were categorized using urban versus rural definitions.18 Distances between the geographic center of the individual’s ZIP code and the individual’s delivery hospital, the closest cardiac hospital, and the closest delivery hospital were calculated using the geodesic distance (i.e., the straight-line distance between points) between the centers of the ZIP codes.
The criteria promulgated by the United States Centers for Disease Control and Prevention Severe Maternal Morbidity Criteria were used to identify adverse maternal outcomes of birth from the diagnosis and procedure codes supplied on the SID record.19 We pre-specified a primary composite outcome of cardiac SMM that includes births complicated by acute myocardial infarction, cardiac arrest, need for cardioversion, acute heart failure, pulmonary edema, shock, or mechanical ventilation or tracheostomy; this is a composite outcome we have used previously when evaluating maternal cardiac disease.16 The secondary outcome was the composite non-transfusion SMM. Transfusion secondary to postpartum hemorrhage accounts for a large proportion of overall SMM rates, and the number of units of blood transfused is captured poorly in many facilities in administrative data; therefore, SMM events that only included blood transfusion were excluded in our analysis.20,21 CDC SMM criteria are included in Appendix 2.19
Univariate analysis using the Kruskal-Wallis test for continuous variables and the chi-squared test for binary/categorical variables were performed to compare demographics, comorbid conditions, incidence of SMM, and delivery type across the cardiac disease categories. Logistic regression, adjusting for individual age, primary payor, median ZIP code income, comorbid conditions, and delivery in a cardiac hospital, was used to assess the relationships between rural versus urban living status and outcomes, and race and outcomes. These regression models controlled for a number of comorbid conditions, including gestational diabetes, HIV, pre-gestational diabetes, prior cesarean section, multifetal gestation, asthma, bleeding disorders, Body Mass Index (BMI) greater than 40, chronic kidney disease, autoimmune conditions, previa, substance use disorder, anemia, bariatric surgical status, GI disease, mental health conditions, neuromuscular conditions, abruption, placenta accreta spectrum disorder, and thyroid conditions. Adjustment for hypertensive disorders of pregnancy (chronic hypertension, gestational hypertension, or preeclampsia) is challenging because cardiac disease itself may predispose people to these conditions. For this reason, we assessed the sensitivity of our results to regression both with and without adjustment for these conditions. To determine if observed differences in outcome were driven by facility variation, we additionally performed conditional logistic regression (i.e., fixed effects regression), which allowed us to compare outcomes between Black and White patients, adjusting for the different rates of outcomes at each facility. Because there were missing values of race (2.8%), ZIP code income (0.6%), and primary payor variables (0.4%), multiple imputation was performed using chained equations.22 Statistical analysis was performed in Stata statistical software, Version 16.1. A two-sided alpha value of 0.05 was considered statistically significant. This study was adjudicated to be exempt from review by the Duke University School of Medicine Institutional Review Board, given that the SID is a Limited Data Set as defined by the United States Health Insurance Portability and Accountability Act.
Results
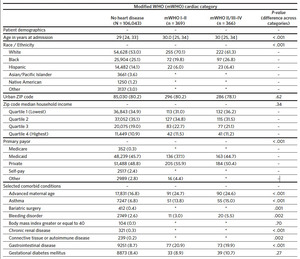
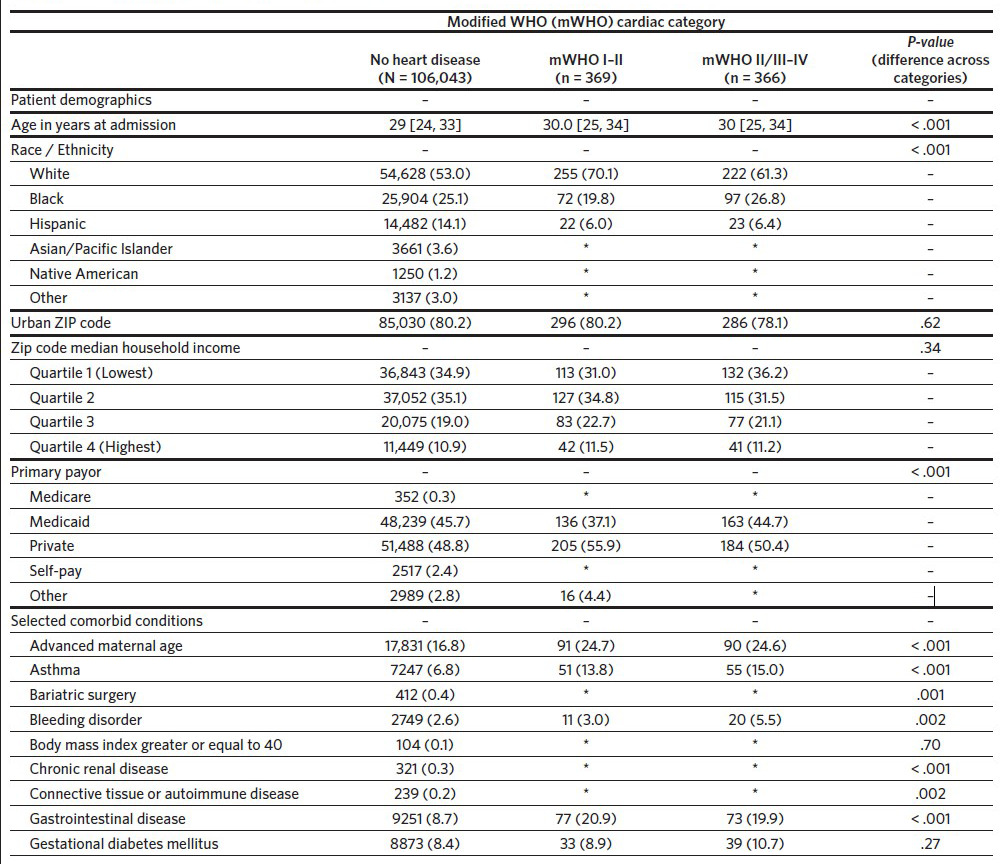
A total of 111,839 delivery hospitalizations were identified. Individuals were excluded who were not North Carolina residents (3185), delivered before 20 weeks (933), or were missing a hospital identifier (943). The cohort thus included 106,778 individuals identified as having a delivery hospitalization in North Carolina in the year 2019. Of these delivery hospitalizations, 106,043 (99.3%) individuals did not have preexisting cardiac disease, 369 (0.3%) had preexisting mWHO category I–II cardiac disease, and 366 (0.3%) women had preexisting mWHO category II/III–IV cardiac disease (Table 1). The prevalence of cardiac disease also differed by race, with White race more common among individuals with preexisting cardiac disease (53.0%, 70.1%, and 61.3% for no cardiac disease, mWHO I–II, and mWHO II/III– IV, respectively; P < .001). Compared to women without cardiac disease, women with preexisting cardiac disease had more comorbid conditions, including chronic hypertension, mental health disorders, and preeclampsia. Individuals with mWHO II/III–IV disease delivered in larger hospitals with a larger annual number of births (3913 versus 3832 versus 3315 for mWHO II/III–IV, mWHO I–II, and no cardiac disease, respectively; P < .001) and were more likely to deliver in cardiac hospitals (76.2% versus 70.2% versus 50.3% for mWHO II/III–IV, mWHO I–II, and no cardiac disease, respectively; P < .001) (Appendix 3). The median distance from the individual’s ZIP code to the ZIP code of the delivery hospital was also longer for those with mWHO II/III–IV disease. Rates of non-transfusion SMM and cardiac SMM were higher for individuals with mWHO II/III–IV disease when compared with those without cardiac disease (10.4% versus 0.1% for cardiac SMM, 13.1% versus 0.6% percent for non-transfusion SMM; P < .001 for both comparisons). Cesarean and preterm delivery rates were also highest for women with cardiac disease (Table 1).
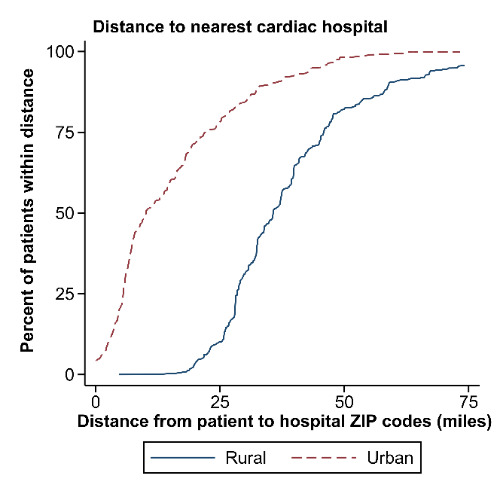
Approximately 19.8% (N = 21,166) of individuals who delivered in North Carolina resided in a rural ZIP code, including 21.8% (n = 88) of individuals with mWHO II/III–IV disease. Residents of rural ZIP codes had longer distances to the ZIP code of the nearest cardiac hospital (median: 35.7 miles, 25th–75th percentiles: 28.2–45.3 miles) when compared with urban residents (median: 10.0 miles, 25th–75th percentiles: 5.6–21.8 miles; P < .001) (Figure 1). Among all deliveries, 22.3% of rural residents delivered in a cardiac hospital versus 57.5% of urban residents; among those patients with mWHO II/III–IV disease, 60.0% of rural residents delivered at a cardiac hospital compared to 80.8% of urban residents (P < .001). There were no differences in the observed rate of cardiac SMM in rural residents when compared to urban residents, both overall and after stratification by mWHO category (Figures 2A and 2B). Differences were also not significant when adjusting using regression models for demographic characteristics or comorbid conditions, and when evaluating the non-transfusion SMM endpoint (results not shown). Among the cohort of patients with mWHO II/ III–IV disease, there was also no difference in the incidence of cardiac SMM based on delivery in a cardiac hospital versus a non-cardiac hospital (11.1% versus 8.1%, P = .41).
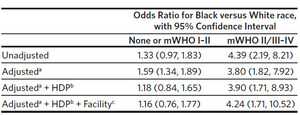
Comparisons of racial and ethnic disparities were restricted to Black and White individuals, because the sample sizes for other racial and ethnic groups in the mWHO II/III–IV group were too small for comparisons. For similar sample size reasons, individuals with no cardiac disease and mWHO I–II disease were consolidated into a single category and compared to those with mWHO II/III–IV disease. This design resulted in a sample of 81,878 individuals identifying as either Black or White. Among these individuals, rates of cardiac SMM were higher for Black individuals than White (0.28% versus 0.13%, P < .001) (Figure 3A). The racial disparity in cardiac SMM rates was larger, both on an absolute (incremental) risk difference and on a relative risk difference, for individuals with mWHO II/III–IV disease than for those having no cardiac disease or mWHO I/II disease (Figure 3B). These differences persisted after adjustment (Table 2).
Discussion
In this retrospective cohort study, we utilized a statewide database of births across North Carolina in 2019 to characterize the volume of births by individuals with known cardiac disease and the potential for rural/urban and racial disparities in adverse cardiac outcomes. We verified that individuals with mWHO II/III–IV disease have a higher risk of cardiac complications than do individuals with no preexisting maternal cardiac disease or low-risk lesions (mWHO I–II).23 While there is a disparity in the capabilities of facilities in which urban and rural residents with high-risk lesions deliver, this does not result in a statistically significant difference in cardiac SMM. In contrast, for Black individuals when compared to White individuals, the overall rate of cardiac complications of birth are significantly higher, and this difference, whether measured as an additive risk or a multiplicative risk, is greater for individuals with high-risk cardiac conditions than those with no or low-risk cardiac conditions.
Current guidelines suggest that individuals with mWHO category II/III–IV disease should deliver at a hospital with advanced cardiovascular capabilities.24 Our study suggests that despite these recommendations, approximately a quarter of this high-risk population does not deliver at a cardiac facility. There are likely differences in patients with mWHO II/III–IV conditions who deliver at cardiac facilities versus those who do not, potentially driven by differences in access to care and patient preferences. While the results did not demonstrate a difference in outcomes among mWHO II–III/IV patients based on the cardiovascular capability of their delivery facility, we suspect that there are strong, unmeasured selection effects. Additionally, the technologies we identified as being indicative of a cardiac hospital (such as ECMO) are tools that can rescue an individual experiencing a life-threatening complication of birth, but such technologies would not be expected to necessarily prevent the occurrence of the complication. Thus, it is not clear that cardiac SMM rates would necessarily be altered by these technologies, but these technologies would be expected to mitigate the impact of cardiac SMM on survival.
Distance traveled to delivery facility is an important factor to consider when discussing widespread access to prenatal care, especially for high-risk pregnancies. Our results demonstrated that individuals with more severe cardiac disease in pregnancy traveled further distances to their delivery facility. We found that fewer individuals from rural areas delivered at a cardiac facility than did their urban counterparts, yet the overall rates of cardiac SMM did not differ between these populations. As prior studies have demonstrated, longer distances to prenatal care may influence adverse outcomes in pregnancy.25–27 Although we did not identify differences in outcomes based on distance traveled or delivery facility, we were limited by relatively small sample sizes in our evaluation of SMM at delivery, particularly in the rural population with mWHO II/III–IV disease. Further investigation might reveal that delayed access to care has a potential impact on SMM events in the antepartum or postpartum period.
Prior studies have identified a higher incidence of cardiac and non-cardiac SMM in women of racial or ethnic minority status compared with White individuals.11,28 In our study, we illustrate the important role of preexisting cardiac disease in mediating this disparity, suggesting that improving the pregnancy care that Black individuals in North Carolina with preexisting cardiac disease receive is a possible intervention to reduce racial disparities in maternal outcomes. Standardization of care is a powerful tool for improving maternal outcomes and reducing racial inequalities.29 For patients with complex maternal cardiac disease, the advent of Pregnancy Heart Teams (with a multidisciplinary, standardized approach to these complex patients) is the new standard of care.30–32 The creation of regional Centers of Excellence, with the development of Pregnancy Heart Teams that focus on improving maternal cardiovascular care, should be considered as a next step toward improving care of individuals with cardiovascular disease in North Carolina.
This study has several limitations. First, the North Carolina SID does not include readmissions. We are unable to capture up to 22% of new SMM events that occur in the postpartum period, which are two-fold more likely to occur within the first 42 days postpartum in individuals with any SMM event at their delivery admission.33,34 This limitation also resulted in our inability to study antepartum admissions, which might have captured a broader SMM rate for the study population of women with cardiac disease in pregnancy. Our delivery distance information was based on geographic centers of ZIP codes, which is an imprecise measure and does not correlate perfectly with transportation time. Additionally, despite including virtually all births in North Carolina during 2019, the numbers of rural residents with mWHO II/III–IV disease was low and may be insufficient to identify differences in outcomes.
The most important limitation of this study is our dependence on diagnosis and procedure codes for identification of deliveries, cardiac disease, comorbidities, and outcomes. The sensitivity and specificity of ICD-10-CM codes is variable and is likely tied to the quality of provider documentation and whether the addition of a specific diagnosis or procedure code alters facility reimbursement. For example, the prevalence of BMI of 40 or greater reported in this study (0.1%) does not correlate with our clinical experience or that of the original publication of the expanded Obstetric Comorbidity Index, where the prevalence was 4.1%. This study may not reflect the full burden of preexisting cardiac disease prior to birth if individuals were not coded as having a cardiac disease. This problem may be even greater in individuals of lower socioeconomic status who face barriers to care and thus have lower health care utilization. Therefore, our estimates potentially underestimate the true degree of disparity in North Carolina. Consideration of a high-quality, prospective database of patients who are pregnant with maternal cardiac disease in North Carolina has the potential to better describe, track, and measure quality and outcomes of this critically important population.
Conclusion
In summary, this study reports on the volume of delivery hospitalizations with preexisting maternal cardiac disease in North Carolina and demonstrates considerable racial disparities in cardiac SMM in pregnant individuals with moderate to severe cardiac disease in this state. While statistically significant differences in outcome were not noted, there was a statistically significant reduction in the number of rural patients with cardiovascular disease who delivered at a facility equipped to provide comprehensive cardiac care. These findings can inform counseling of high-risk individuals with cardiac disease and birth planning and may also assist in developing systems of care to provide evidence-based, high-quality care for these patients.
–
Acknowledgments
Financial support. Dr. Federspiel is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Meng is supported by a Mentored Research Training Grant from the Foundation for Anesthesia Education and Research.
The North Carolina State Inpatient Database is a product of the Healthcare Cost and Utilization Project of the United States Agency for Healthcare Research and Quality, in concert with the North Carolina Department of Health and Human Services.
This project was supported by the National Center for Advancing Translational Sciences through Grant Award TL1TR002555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This paper was presented in preliminary form at the 2022 NC Obstetrical and Gynecological Society Meeting (April 8-10, 2022; Kiawah Island, South Carolina).